Middle East and Africa Gene Therapy Market Analysis and Insights
Middle East and Africa gene therapy market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services and products. Along with this, manufacturers are engaged in R&D activity for launching novel products in the market.
The increasing research in gene therapy and its techniques and development is expected to boost market growth further. However, the ethical and safety concerns while performing the method is expected to hamper the growth of the Middle East and Africa gene therapy market in the forecast period. Increasing demand for gene therapy, an emerging and advanced field in genetic engineering and healthcare, is expected to give opportunities to the market to enhance the treatment and diagnostic approaches.
The growing demand for better quality healthcare for cancers and genetic disorders is expected to boost the market’s growth. However, the high cost of diagnostics and the lack of skilled and certified professionals are expected to challenge market growth.
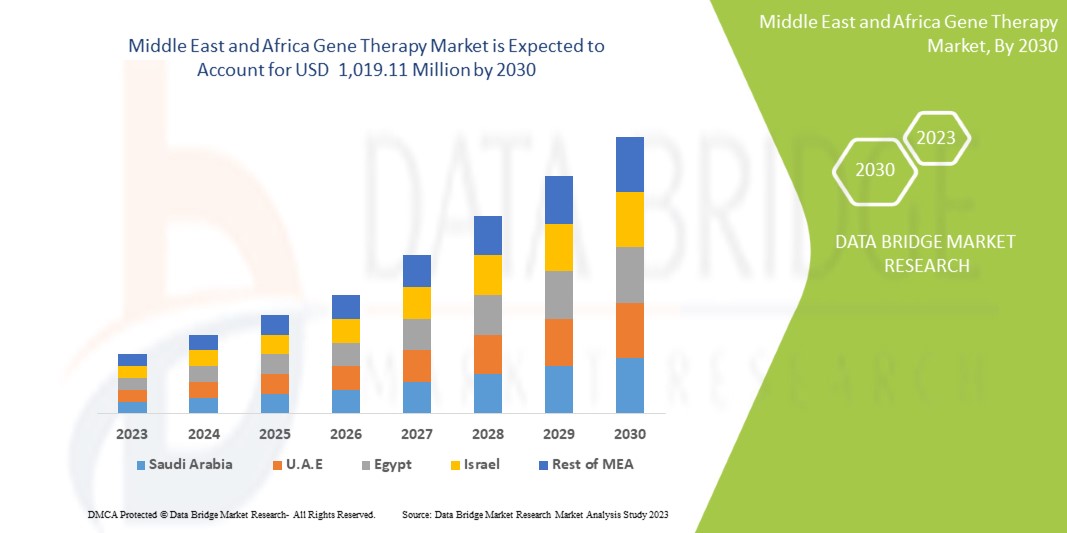
Data Bridge Market Research analyzes that the Middle East and Africa gene therapy market is expected to reach the value of USD 1,019.11 million by 2030, at a CAGR of 16.6% during the forecast period. Product accounts for the largest type segment in the market due to growing use of exosome research products in diagnostics and therapeutics.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
By Vector Type (Viral Vector and Non-viral Vector), Method (Ex-vivo and In-vivo), Application (Oncological Disorders, Cardiovascular Diseases, Infectious Diseases, Rare Diseases, Neurological Disorders, and Other Diseases), End User (Cancer Institutes, Hospitals, Research Institutes, and Others) |
|
Countries Covered |
South Africa and Rest of Middle East and Africa |
|
Market Players Covered |
Novartis AG, Kite Pharma (a subsidiary of Gilead Sciences, Inc.), uniQure NV., Oxford Biomedica, Spark Therapeutics, Inc.. SIBONO, bluebird bio, Inc., Shanghai Sunway Biotech Co., Ltd., Biogen, Dendreon Pharmaceuticals LLC., Amgen Inc., AnGes, Inc., and Enzyvant Therapeutics GmbH among others |
Middle East and Africa Gene Therapy Market Definition
Gene therapy is a medical strategy that addresses the underlying genetic issue in order to treat or prevent disease. Instead of utilising drugs or surgery, gene therapy procedures allow doctors to treat a problem by changing a person's genetic composition. A select few disorders, including an eye condition called Leber congenital amaurosis and a muscle condition called spinal muscular atrophy, are being treated with gene therapy. To ensure that they will be secure and efficient, many other gene therapies are undergoing study. Medical professionals aim to soon apply the promising technique of genome editing to cure human illnesses.
The ability to successfully transport a therapeutic gene to a target cell is the most important prerequisite for gene therapy to be successful. Once transported, that gene must go to the cell wall's nucleus, where it will serve as a model for making protein molecules. The principal therapeutic action is then produced by the protein. For instance, cell destruction might be used in the treatment of tumours, whereas cell preservation might be used in the case of neurodegenerative illness. However, strict regulations and standards for the approval and commercialization of products is expected to restrain the market growth.
Middle East and Africa Gene Therapy Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Novel approaches of gene therapy
Gene therapy has brought permanent cures for ailments that were previously simply temporary treatments. For a very long period, gene therapy did not work; however, in recent years, effective and long-lasting treated cases have been recorded. For a wide range of hereditary illnesses, including blood abnormalities, immunological deficiencies, vision issues, nerve cell regeneration, metabolic disorders, and different types of cancer, promising outcomes have been attained.
With more specificity and fewer side effects, gene therapy has the potential to be a customized medicine that can "cure" a variety of diseases. Gene therapy generally refers to the transfer of genetic material to treat an illness, or at the very least, to enhance a patient's clinical condition. Using viruses as genetic vectors to deliver the desired gene to the target cells is one method of how gene therapy functions. These vectors are classified as RNA-based or DNA-based viral vectors depending on the type of genome they contain.
The majority of experts concur that gene therapy has the potential to be the most intriguing use of DNA research to date. A simple intravenous injection of a gene transfer agent may one day be used to administer genes as medicine, seeking out target cells for stable, site-specific chromosomal integration and subsequent gene expression. It is predicted that there would be a need for gene therapy using revolutionary techniques that are being tested by researchers around the world and incorporated into conventional treatment is expected to act as driver for the growth of Middle East and Africa gene therapy market
- Increasing prevalance of genetic disorders
A sizable share of prenatal and neonatal mortality in several nations in the Region is caused by genetic and congenital diseases. Many multifactorial ailments are often caused by genetic factors as well. Gene alterations that are essentially present in every cell in the body cause many hereditary diseases. These illnesses thus frequently impact many bodily systems, and the majority cannot be treated.
For instance,
- The Department of Health indicated that roughly six out of ten people will be impacted by an ailment that has some genetic ties, according to the Government of Western Australia. Genetic disorders can range from minor to very severe. Between 3 and 5% of newborns born in Western Australia have genetic disorders or birth abnormalities
Mutations, exposure to chemicals and radiation, among other things, can all result in genetic disorders. Although some ailments have been treated with gene therapy, the majority of treatment plans for genetic disorders do not change the underlying genetic abnormality. For this, the prevalence of genetic abnormalities is significantly rising across all age groups and practically all geographic areas are expected to act as a driver for the growth of Middle East and Africa gene therapy market.
Restraint
- High cost of gene therapy
A new line of medical treatments called gene therapy involves replacing, deleting, or introducing genetic information into a patient's genome in order to treat a condition. Even though it is still in its infancy, gene therapy has already shown tremendous promise for the treatment and even cure of once-intractable illnesses. Gene therapy pricing is still highly uncontrolled and determined on a case-by-case basis in many nations, frequently focusing on a single upfront payment.
For instance,
- According to a news article published in Web MD in February 2023, Hemgenix is breaking records but is hardly an anomaly. In September 2022, Skysona, a medication for a rare neurological condition, went on sale for $3 million. Just one month prior, Zynteglo, a gene treatment for a genetic blood condition, made its market debut for $2.8 million. A cure for the genetic condition spinal muscular atrophy, which kills infants and young children, called Zolgensma cost $2.1 million in 2019.
Even if they are not always fully curative, gene therapies can be really transforming. The main barrier to accessing gene therapy is cost. The treatment landscape for many rare genetic illnesses will undergo significant change as additional gene therapy products become available, providing patients with potentially curative alternatives for the first time. The difficulty of making sure that all patients, not just a small group with financial means and privileged access to technology, may benefit from these cutting-edge therapies must be addressed by healthcare systems around the world. However, due to the high cost of the treatments
Opportunity
-
Rise in strategic acquisition and partnership among organizations
Recently, different organizations are stepping forward for partnership and collaboration to develop various gene therapy products that are essential for detecting genetic disorders. Not only this, with the help of partnerships and agreements both the companies can develop a new suite of technologies and platforms that will help to detect diseases.
With the help of a long-term agreement, both companies can provide dimensional pricing of gene therapy products in response to consumer demand in the market. Such partnership and mutual agreement not only benefit both the companies but are also creating a lot of opportunities for the market to grow.
For instance,
-
In July 2022, Novartis Pharmaceuticals UK announces the launch of the Novartis Biome UK Heart Health Catalyst 2022, in a world-first investor partnership with Medtronic ltd, RYSE Asset Management, and Chelsea and Westminster Hospital NHS Foundation Trust and its official charity CW+
Therefore, a rise in collaboration & partnerships is further expected to create a lot of opportunities for the market to grow
Challenge
- Stringent regulations for gene therapy products
The use of gene therapy across the globe is rapidly increasing, with the growth of the aged population and several chronic diseases which are preventable by early diagnosis and timely treatments. At the same time, the players of the gene therapy in the market have to follow certain regulations to get approval from the upper authorities for the launching of the product in a region. These stringent guidelines need to be followed, and this is one of the most difficult tasks among all the steps. The pre-market approval of various gene therapy products varies from one country to another.
For instance,
- In 2022, according to the information provided by Food and Drug Administration (FDA), the Center for Biologics Evaluation and Research (CBER) regulates cellular therapy products, human gene therapy products, and certain devices related to cell and gene therapy. CBER uses both the Public Health Service Act and the Federal Food Drug and Cosmetic Act as enabling statutes for oversight.
Hence, stringent regulations for gene therapy products is different for different countries which is expected to act as a challenge in the market growth.
Recent Developments
- In December 2022 Kite Pharma, Inc., and Daiichi Sankyo Co., Ltd. announced that the Japan Ministry of Health, Labour and Welfare (MHLW) has approved Yescarta (axicabtagene ciloleucel), a chimeric antigen receptor (CAR) T-cell therapy, for the initial treatment of patients with relapsed/refractory large B-cell lymphoma (R/R LBCL): diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, transformed follicular lymphoma, and high-grade B-cell lymphoma. Only patients who have not previously had a transfusion of CAR T cells directed against the CD19 antigen should be treated with Yescarta
- In December, Ferring Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved Adstiladrin (nadofaragene firadenovec-vncg), a novel adenovirus vector-based gene therapy, for the treatment of adult patients with high-risk, Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors. This has helped the company to expand their product portfolio
Middle East and Africa Gene Therapy Market Scope
The Middle East and Africa gene therapy market is segmented into four notable segments based on vector type, method, application, and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
BY VECTOR TYPE
- Viral Vector
- Non-Viral Vector
On the basis of vector type, the Middle East and Africa gene therapy market is segmented into viral and non-viral vector.
BY METHOD
- Ex-Vivo
- In-Vivo
On the basis of method, the Middle East and Africa gene therapy market is segmented into ex vivo and in vivo.
BY APPLICATION
- Oncological Disorders
- Cardiovascular Diseases
- Infectious Disease
- Rare Diseases
- Neurological Disorders
- Other Diseases
On the basis of application, the Middle East and Africa gene therapy market is segmented into oncological disorders, cardiovascular diseases, infectious diseases, rare diseases, neurological disorders, and other diseases.
BY END USER
- Cancer Institutes
- Hospitals
- Research Institutes
- Others
On the basis of end user, the Middle East and Africa gene therapy market is segmented into cancer institutes, hospitals, research institutes, and others.
Middle East and Africa Gene Therapy Market Regional Analysis/Insights
The Middle East and Africa gene therapy market is segmented into four notable segments based on vector type, method, application, and end user.
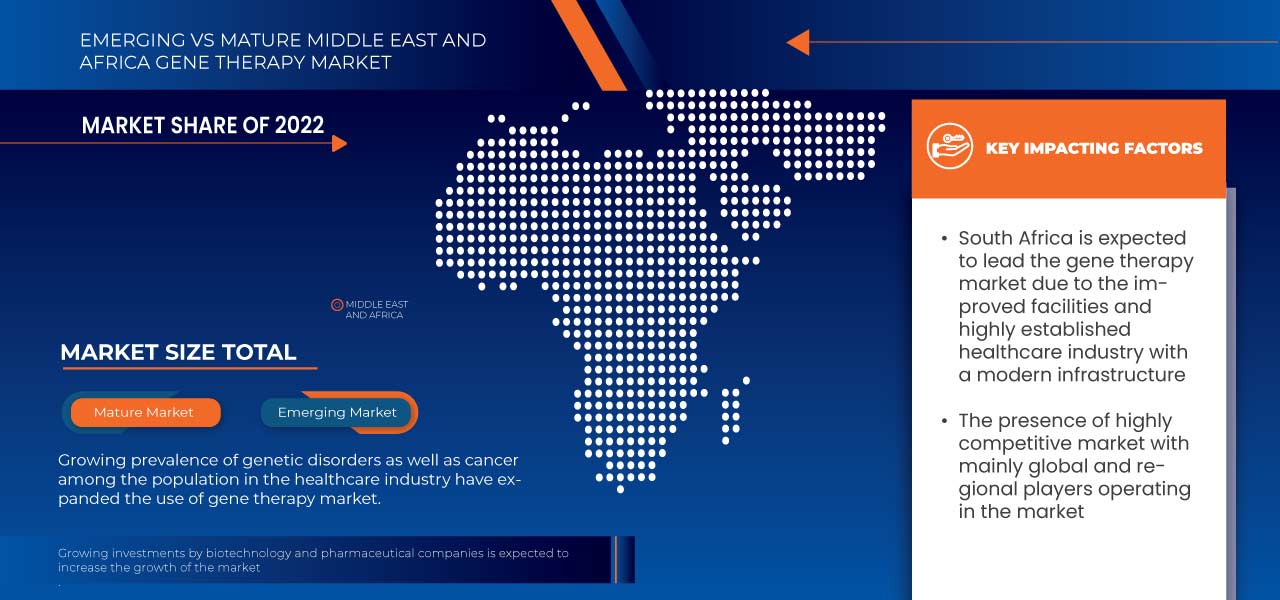
The countries covered in this market report are South Africa and the rest of the Middle East and Africa.
South Africa is dominating due to the presence of key market players in the largest consumer market with high GDP.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the significant pointers used to forecast the market scenario for individual countries. Also, presence and availability of Middle East and Africa brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East and Africa Gene Therapy Market Share Analysis
Middle East and Africa gene therapy market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breath, application dominance, product type lifeline curve. The above data points provided are only related to the company’s focus on the Middle East and Africa gene therapy market.
Some of the major players operating in the Middle East and Africa gene therapy market are:, Novartis AG, Kite Pharma (a subsidiary of Gilead Sciences, Inc.), uniQure NV, Oxford Biomedica, Spark Therapeutics, Inc., SIBONO, bluebird bio, Inc., Shanghai Sunway Biotech Co. Ltd., Biogen, Dendreon Pharmaceuticals LLC., Amgen Inc., AnGes, Inc. and Enzyvant Therapeutics GmbHAudubon Bioscience, among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST & AFRICA GENE THERAPY MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER FIVE ANALYSIS
5 UPDATE ON GERMLINE GENE THERAPY
5.1 GERMLINE GENE THERAPY
6 MIDDLE EAST & AFRICA GENE THERAPY MARKET, MO
6.1 DRIVERS
6.1.1 NOVEL APPROACHES TO GENE THERAPY
6.1.2 INCREASING PREVALENCE OF GENETIC DISORDERS
6.1.3 THE GROWING INVESTMENT BY BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES
6.1.4 GROWING DEMAND FOR PERSONALIZED MEDICINE
6.2 RESTRAINTS
6.2.1 HIGH COST OF GENE THERAPY
6.2.2 ETHICAL AND SAFETY CONCERNS
6.2.3 COMPLEXITY OF GENE THERAPY
6.3 OPPORTUNITIES
6.3.1 RISE IN STRATEGIC ACQUISITION AND PARTNERSHIP AMONG ORGANIZATIONS
6.3.2 RISING APPROVAL FOR GENE THERAPY PRODUCTS
6.4 CHALLENGES
6.4.1 STRINGENT REGULATIONS FOR GENE THERAPY PRODUCTS
6.4.2 LONG-TERM SAFETY AND EFFICACY
7 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY VECTOR TYPE
7.1 OVERVIEW
7.2 VIRAL VECTOR
7.2.1 ADENOVIRUS
7.2.2 RETROVIRUS
7.2.3 LENTIVIRUS
7.2.4 ADENO-ASSOCIATED VIRUS
7.2.5 VACCINIA VIRUS
7.2.6 HERPES SIMPLEX VIRUS
7.2.7 OTHERS
7.3 NON-VIRAL VECTOR
7.3.1 LIPOFECTION
7.3.2 INJECTION OF NAKED DNA
8 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY METHOD
8.1 OVERVIEW
8.2 EX-VIVO
8.3 IN-VIVO
9 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 ONCOLOGICAL DISORDERS
9.3 CARDIOVASCULAR DISEASES
9.4 INFECTIOUS DISEASES
9.5 RARE DISEASES
9.6 NUEROLOGICAL DISORDERS
9.7 OTHER DISEASES
10 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY END USER
10.1 OVERVIEW
10.2 CANCER INSTITUTES
10.3 HOSPITALS
10.4 RESEARCH INSTITUTES
10.5 OTHERS
11 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY REGION
11.1 MIDDLE EAST AND AFRICA
11.1.1 SOUTH AFRICA
11.1.2 REST OF MIDDLE EAST AND AFRICA
12 MIDDLE EAST & AFRICA GENE THERAPY MARKET, COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
13 COMPANY PROFILES
13.1 BIOGEN
13.1.1 COMPANY SNAPSHOT
13.1.2 REVENUE ANALYSIS
13.1.3 COMPANY SHARE ANALYSIS
13.1.4 SWOT ANALYSIS
13.1.5 PRODUCT PORTFOLIO
13.1.6 RECENT DEVELOPMENT
13.2 KITE PHARMA
13.2.1 COMPANY SNAPSHOT
13.2.2 REVENUE ANALYSIS
13.2.3 COMPANY SHARE ANALYSIS
13.2.4 SWOT ANALYSIS
13.2.5 PRODUCT PORTFOLIO
13.2.6 RECENT DEVELOPMENT
13.3 NOVARTIS AG
13.3.1 COMPANY SNAPSHOT
13.3.2 REVENUE ANALYSIS
13.3.3 COMPANY SHARE ANALYSIS
13.3.4 SWOT ANALYSIS
13.3.5 PRODUCT PORTFOLIO
13.3.6 RECENT DEVELOPMENTS
13.4 BRISTOL-MYERS SQUIBB COMPANY.
13.4.1 COMPANY SNAPSHOT
13.4.2 REVENUE ANALYSIS
13.4.3 COMPANY SHARE ANALYSIS
13.4.4 SWOT ANALYSIS
13.4.5 PRODUCT PORTFOLIO
13.4.6 RECENT DEVELOPMENT
13.5 OXFORD BIOMEDICA
13.5.1 COMPANY SNAPSHOT
13.5.2 REVENUE ANALYSIS
13.5.3 COMPANY SHARE ANALYSIS
13.5.4 SWOT ANALYSIS
13.5.5 PRODUCT PORTFOLIO
13.5.6 RECENT DEVELOPMENTS
13.6 AGC BIOLOGICS
13.6.1 COMPANY SNAPSHOT
13.6.2 PRODUCT PORTFOLIO
13.6.3 RECENT DEVELOPMENT
13.7 ANGES, INC
13.7.1 COMPANY SNAPSHOT
13.7.2 REVENUE ANALYSIS
13.7.3 PRODUCT PORTFOLIO
13.7.4 RECENT DEVELOPMENT
13.8 AMGEN INC.
13.8.1 COMPANY SNAPSHOT
13.8.2 REVENUE ANALYSIS
13.8.3 PRODUCT PORTFOLIO
13.8.4 RECENT DEVELOPMENT
13.9 BLUEBIRD BIO, INC.
13.9.1 COMPANY SNAPSHOT
13.9.2 PRODUCT PORTFOLIO
13.9.3 RECENT DEVELOPMENT
13.1 CHIESI FARMACEUTICI S.P.A
13.10.1 COMPANY SNAPSHOT
13.10.2 REVENUE ANALYSIS
13.10.3 PRODUCT PORTFOLIO
13.10.4 RECENT DEVELOPMENT
13.11 DENDREON PHARMACEUTICALS LLC
13.11.1 COMPANY SNAPSHOT
13.11.2 PRODUCT PORTFOLIO
13.11.3 RECENT DEVELOPMENT
13.12 ENZYVANT THERAPEUTICS GMBH
13.12.1 COMPANY SNAPSHOT
13.12.2 RODUCT PORTFOLIO
13.12.3 RECENT DEVELOPMENT
13.13 FERRING B.V.
13.13.1 COMPANY SNAPSHOT
13.13.2 PRODUCT PORTFOLIO
13.13.3 RECENT DEVELOPMENT
13.14 JANSSEN PHARMACEUTICALS, INC.
13.14.1 COMPANY SNAPSHOT
13.14.2 PRODUCT PORTFOLIO
13.14.3 RECENT DEVELOPMENT
13.15 MALLINCKRODT.
13.15.1 COMPANY SNAPSHOT
13.15.2 REVENUE ANALYSIS
13.15.3 PRODUCT PORTFOLIO
13.15.4 RECENT DEVELOPMENT
13.16 ORCHARD THERAPEUTICS PLC.
13.16.1 COMPANY SNAPSHOT
13.16.2 REVENUE ANALYSIS
13.16.3 PRODUCT PORTFOLIO
13.16.4 RECENT DEVELOPMENT
13.17 SHANGHAI SUNWAY BIOTECH CO., LTD.
13.17.1 COMPANY SNAPSHOT
13.17.2 PRODUCT PORTFOLIO
13.17.3 RECENT DEVELOPMENT
13.18 SIBONO
13.18.1 COMPANY SNAPSHOT
13.18.2 PRODUCT PORTFOLIO
13.18.3 RECENT DEVELOPMENT
13.19 SPARK THERAPEUTICS, INC.
13.19.1 COMPANY SNAPSHOT
13.19.2 PRODUCT PORTFOLIO
13.19.3 RECENT DEVELOPMENT
13.2 UNIQURE NV.
13.20.1 COMPANY SNAPSHOT
13.20.2 REVENUE ANALYSIS
13.20.3 PRODUCT PORTFOLIO
13.20.4 RECENT DEVELOPMENT
14 QUESTIONNAIRE
15 RELATED REPORTS
List of Table
TABLE 1 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA VIRAL VECTOR IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA VIRAL VECTOR IN GENE THERAPY MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA NON-VIRAL VECTOR IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA NON-VIRAL VECTOR IN GENE THERAPY MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY METHOD, 2021-2030 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA EX-VIVO IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA IN –VIVO IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA ONCOLOGICAL DISORDERS IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CARDIOVASCULAR DISEASES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA INFECTIOUS DISEASES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA RARE DISEASES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA NEUROLOGICAL DISORDERS IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA OTHER DISEASES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA GENE THERAPY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA CANCER INSTITUTES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA HOSPITALS IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA RESEARCH INSTITUTES IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA OTHERS IN GENE THERAPY MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
TABLE 23 MIDDLE EAST AND AFRICA VIRAL VECTOR IN GENE THERAPY MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 24 MIDDLE EAST AND AFRICA NON-VIRAL VECTOR IN GENE THERAPY MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY METHOD, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 28 SOUTH AFRICA GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
TABLE 29 SOUTH AFRICA VIRAL VECTOR IN GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
TABLE 30 SOUTH AFRICA NON-VIRAL VECTOR IN GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
TABLE 31 SOUTH AFRICA GENE THERAPY MARKET, BY METHOD, 2021-2030 (USD MILLION)
TABLE 32 SOUTH AFRICA GENE THERAPY MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 33 SOUTH AFRICA GENE THERAPY MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 REST OF MIDDLE EAST AND AFRICA GENE THERAPY MARKET, BY VECTOR TYPE, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 MIDDLE EAST & AFRICA GENE THERAPY MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA GENE THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA GENE THERAPY MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA GENE THERAPY MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA GENE THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA GENE THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA GENE THERAPY MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA GENE THERAPY MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA GENE THERAPY MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA GENE THERAPY MARKET: SEGMENTATION
FIGURE 11 THE INCREASING PREVALENCE OF GENETIC DISORDERS AND GROWING DEMAND FOR PERSONALIZED MEDICINE ARE EXPECTED TO DRIVE THE GROWTH OF THE MIDDLE EAST & AFRICA GENE THERAPY MARKET FROM 2023 TO 2030
FIGURE 12 THE VIRAL VECTOR SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA GENE THERAPY MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA GENE THERAPY MARKET
FIGURE 14 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY VECTOR TYPE, 2022
FIGURE 15 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY VECTOR TYPE, 2023-2030 (USD MILLION)
FIGURE 16 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY VECTOR TYPE, CAGR (2023-2030)
FIGURE 17 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY VECTOR TYPE, LIFELINE CURVE
FIGURE 18 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY METHOD, 2022
FIGURE 19 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY METHOD, 2023-2030 (USD MILLION)
FIGURE 20 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY METHOD, CAGR (2023-2030)
FIGURE 21 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY METHOD, LIFELINE CURVE
FIGURE 22 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY APPLICATION, 2022
FIGURE 23 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 24 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 25 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 26 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY END USER, 2022
FIGURE 27 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 MIDDLE EAST & AFRICA GENE THERAPY MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 MIDDLE EAST AND AFRICA GENE THERAPY MARKET: SNAPSHOT (2022)
FIGURE 31 MIDDLE EAST AND AFRICA GENE THERAPY MARKET: BY COUNTRY (2022)
FIGURE 32 MIDDLE EAST AND AFRICA GENE THERAPY MARKET: BY COUNTRY (2023 & 2030)
FIGURE 33 MIDDLE EAST AND AFRICA GENE THERAPY MARKET: BY COUNTRY (2022 & 2030)
FIGURE 34 MIDDLE EAST AND AFRICA GENE THERAPY MARKET: VECTOR TYPE (2023-2030)
FIGURE 35 MIDDLE EAST & AFRICA GENE THERAPY MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.