Global Vaccine Production Market
Market Size in USD Billion
CAGR :
% 
 USD
54.77 Billion
USD
95.88 Billion
2024
2032
USD
54.77 Billion
USD
95.88 Billion
2024
2032
| 2025 –2032 | |
| USD 54.77 Billion | |
| USD 95.88 Billion | |
|
|
|
|
Vaccine Production Market Size
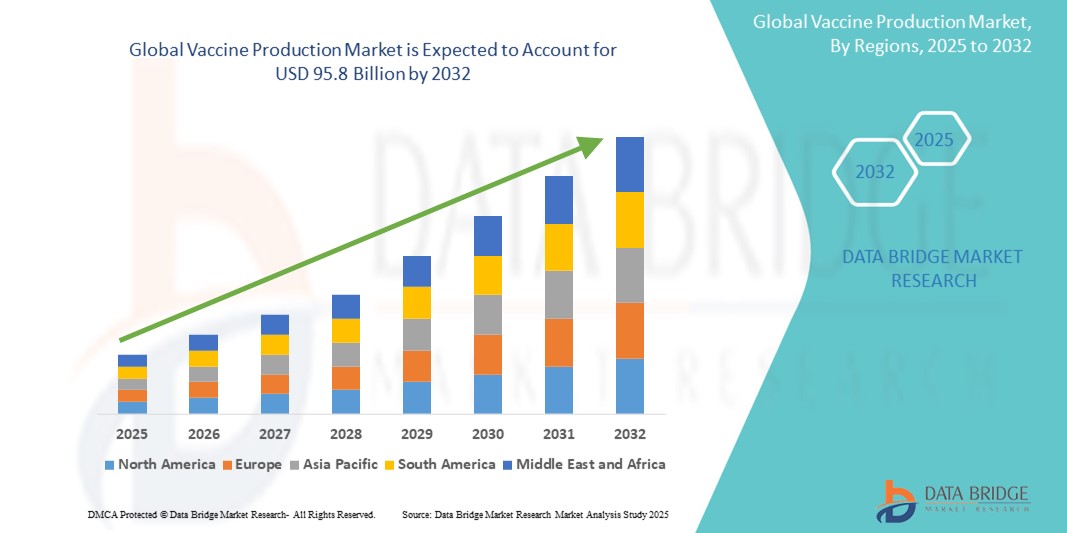
- The global vaccine production market size was valued at USD 54.77 billion in 2024 and is expected to reach USD 95.88 billion by 2032, at a CAGR of7.25% during the forecast period
- The Vaccine Production Market is experiencing significant growth, primarily driven by rapid technological advancements and increasing global demand for effective immunization solutions. This expansion is leading to greater digitalization throughout the vaccine value chain, from research and development to manufacturing and distribution
- Furthermore, rising global health consciousness and the imperative for swift responses to emerging infectious diseases are establishing advanced vaccine production solutions as the modern standard for public health preparedness. These converging factors are accelerating the uptake of innovative Vaccine Production solutions, thereby significantly boosting the industry's growth
Vaccine Production Market Analysis
- The vaccine production market is experiencing significant growth, driven by the escalating global demand for effective immunization solutions and rapid technological advancements, leading to increased digitalization across the entire vaccine value chain—from research and development to manufacturing and distribution. This expansion highlights the critical role of vaccines in modern public health and disease prevention
- The escalating demand for vaccine production solutions is primarily fuelled by the increasing prevalence of infectious diseases, heightened global health security concerns, and a rising emphasis on preventive healthcare. Furthermore, significant breakthroughs in vaccine technologies, such as mRNA and viral vector platforms, are accelerating development and production capabilities, establishing advanced vaccine production solutions as the modern standard for public health preparedness
- North America dominated the vaccine production market, with the largest revenue share of 43% in 2024. This dominance is attributed to early and strong adoption of advanced healthcare technologies, high disposable incomes, and the robust presence of key industry players, including major pharmaceutical and biotechnology companies
- Asia-Pacific is expected to be the fastest-growing region with a CAGR of 12.4% in the vaccine production market during the forecast period. This growth is largely due to increasing urbanization, rising disposable incomes, expanding healthcare infrastructure, and a growing focus on public health initiatives and immunization programs in countries such as China and India, which are also becoming significant vaccine manufacturing hubs
- Adult Vaccine Production segment dominated the vaccine production market with a market share of 58.3% in 2024, driven by increasing demand for vaccines targeting adult populations such as influenza, pneumococcal, and shingles
Report Scope and Vaccine Production Market Segmentation
|
Attributes |
Vaccine Production Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Vaccine Production Market Trends
“Vaccine Production Market Dynamics: Evolving Landscape and Strategic Imperatives”
- A significant and accelerating trend in the global vaccine production market is the continuous evolution of manufacturing platforms and the increasing focus on rapid response capabilities. This advancement in production technologies is fundamentally enhancing the speed and scale at which new vaccines can be developed and manufactured, critically impacting global public health preparedness
- For instance, the rapid development and deployment of mRNA vaccine platforms during recent global health crises demonstrated their ability to accelerate vaccine availability. Similarly, advancements in viral vector and recombinant protein technologies are contributing to a diverse pipeline of vaccine candidates, offering versatile solutions for various infectious diseases
- The integration of advanced bioprocessing technologies, such as single-use systems and continuous manufacturing, enables features such as improved flexibility and reduced contamination risks in vaccine production. For instance, some leading manufacturers utilize advanced bioreactors and purification systems to optimize yield and ensure high-quality vaccine outputs. Furthermore, the adoption of modular manufacturing units offers the ease of rapid deployment and scalability, allowing for decentralized production and quicker global distribution
- The seamless integration of research, development, and manufacturing processes facilitates centralized control over various aspects of the vaccine value chain. Through a unified approach, stakeholders can manage R&D pipelines alongside clinical trials, regulatory submissions, and large-scale production, creating a more efficient and responsive ecosystem
- This trend towards more agile, efficient, and interconnected vaccine production systems is fundamentally reshaping global expectations for disease prevention and pandemic response. Consequently, companies such as BioNTech and Moderna are continuously investing in innovative platform technologies to enhance their production capacity and speed
- The demand for vaccine production solutions that offer seamless scalability and rapid deployment capabilities is growing rapidly across both developed and developing nations, as governments and healthcare organizations increasingly prioritize preparedness and comprehensive immunization strategies
Vaccine Production Market Dynamics
Driver
“Growing Need Due to Rising Global Health Threats and Technological Advancements”
- The increasing prevalence of infectious diseases, coupled with the accelerating pace of biotechnological advancements, is a significant driver for the heightened demand for vaccine production. This includes the emergence of novel pathogens and the re-emergence of older ones, requiring continuous innovation in vaccine development and manufacturing
- For instance, the ongoing global preparedness efforts post-COVID-19 have led to substantial investments in pandemic readiness, including expanding vaccine manufacturing capabilities worldwide. Such strategic initiatives by governments and pharmaceutical companies are expected to drive the Vaccine Production industry's growth in the forecast period
- As nations become more aware of potential public health threats and seek enhanced protection for their populations, advanced vaccine production technologies offer features such as rapid scalability, diverse platform options, and improved efficacy, providing a compelling upgrade over traditional, slower manufacturing methods
- Furthermore, the growing global focus on preventive healthcare and the desire for equitable access to life-saving vaccines are making robust vaccine production an integral component of national health strategies, offering seamless integration with broader public health initiatives and emergency response plans
- The convenience of faster development cycles, the ability to rapidly produce large quantities of vaccines, and the potential for platform technologies to adapt to new variants are key factors propelling the expansion of vaccine production in both established and emerging markets. The trend towards distributed manufacturing models and the increasing availability of sophisticated bioprocessing options further contributes to market growth
Restraint/Challenge
“Concerns Regarding Manufacturing Complexities and High Initial Investment”
- Concerns surrounding the immense complexity and high initial capital expenditure required for establishing and scaling vaccine manufacturing facilities pose a significant challenge to broader market penetration. As vaccine production relies on highly specialized equipment, stringent quality control, and sophisticated biological processes, it is susceptible to technical challenges and significant financial outlays, raising anxieties among potential investors and new entrants
- For instance, high-profile reports of manufacturing delays or failures during the scale-up of novel vaccine candidates have made some stakeholders hesitant to commit to large-scale, risky investments in new production capacities
- Addressing these manufacturing complexities through robust process optimization, technology transfer, and collaborative partnerships is crucial for building trust and ensuring reliable supply chains. Companies such as Lonza and Catalent emphasize their advanced CDMO (Contract Development and Manufacturing Organization) capabilities and quality assurance protocols in their marketing to reassure potential clients
- In addition, the relatively high initial cost of some advanced vaccine production facilities and the specialized workforce required can be a barrier to entry for smaller biotech companies or developing regions, particularly where public funding is limited. While basic fill-and-finish capabilities may be more accessible, premium features such as integrated upstream and downstream processing for novel platforms often come with a higher price tag
- While governments and international organizations are providing significant funding, the perceived premium for cutting-edge biomanufacturing technology and the long lead times for facility construction can still hinder widespread expansion, especially for those who do not have immediate access to substantial capital or expertise
Vaccine Production Market Scope
The market is segmented on the basis of classification and disease type.
- By Classification
On the basis of classification, the vaccine production market is segmented into adult vaccine production and pediatric vaccine production. The adult vaccine production segment accounted for the largest market revenue share of 58.3% in 2024, driven by increasing demand for vaccines targeting adult populations such as influenza, pneumococcal, and shingles. The rise in aging populations globally and growing awareness of adult immunization programs contribute significantly to this segment's dominance.
The pediatric vaccine production segment is projected to witness the fastest CAGR of 10.7% from 2025 to 2032. Factors such as mandatory immunization schedules, government-led child vaccination programs, and heightened awareness of childhood disease prevention are propelling this segment’s growth.
- By Disease Type
On the basis of disease type, the vaccine production market is segmented into Hepatitis B, malaria, yellow fever, typhoid, rabies, HIV, Cancer, influenza, west Nile, Japanese encephalitis, pneumococcal, rotavirus, DTP, polio, varicella, meningococcal, measles, mumps and rubella (MMR), and tuberculosis. The influenza segment held the largest market revenue share of 18.9% in 2024, driven by seasonal demand, global vaccination initiatives, and the continuous evolution of flu strains requiring updated vaccines. Annual flu immunization campaigns across developed and emerging economies further support the segment’s strong performance.
The Cancer vaccine segment is expected to witness the fastest CAGR of 12.4% from 2025 to 2032, driven by advancements in personalized medicine, growing investments in immunotherapy, and increasing clinical trials targeting HPV and other cancer-related antigens. The rising prevalence of cancer globally and the shift towards preventive oncology are major growth drivers.
Vaccine Production Market Regional Analysis
- North America dominated the vaccine production market with the largest revenue share of 43% in 2024, driven by increased investment in vaccine manufacturing infrastructure, robust R&D activities, and strong government support for immunization programs
- The region benefits from advanced biopharmaceutical capabilities and favorable regulatory frameworks, making it a hub for vaccine development and production
- Moreover, the presence of major pharmaceutical companies and growing awareness about disease prevention contribute significantly to regional market growth
U.S. Vaccine Production Market Insight
The U.S. vaccine production market captured the largest share of 78.9% in the North America vaccine production market in 2024, supported by high vaccine consumption rates, rising public-private partnerships, and substantial funding for vaccine innovation. The country leads in the development and production of advanced vaccines for diseases such as influenza, HPV, and COVID-19. The adoption of novel platforms such as mRNA and increasing stockpiling efforts for pandemic preparedness are further propelling the U.S. market forward.
Europe Vaccine Production Market Insight
The Europe vaccine production market is projected to expand at a CAGR of 8.3% from 2025 to 2032, driven by stringent regulatory standards, government-led immunization initiatives, and increased investment in public health infrastructure. Urbanization and the need for disease prevention in densely populated areas are pushing demand for large-scale vaccine manufacturing. In addition, Europe is focusing on localized production to ensure supply chain resilience and self-reliance in future pandemics.
U.K. Vaccine Production Market Insight
The U.K. vaccine production market is anticipated to grow at a CAGR of 7.9% from 2025 to 2032, due to the strong emphasis on life sciences innovation, post-Brexit investments in domestic vaccine production, and a proactive national immunization program. Strategic collaborations with global pharma companies and academic institutions, along with a surge in demand for pediatric and travel vaccines, are further fueling market expansion.
Germany Vaccine Production Market Insight
The Germany vaccine production market is expected to grow at a CAGR of 8.1% from 2025 to 2032, fueled by strong government support for biotech innovation, digital health adoption, and increasing demand for sustainable vaccine production solutions. The country’s focus on high-quality manufacturing practices and integrated automation in pharmaceutical facilities enhances vaccine output and global competitiveness.
Asia-Pacific Vaccine Production Market Insight
The Asia-Pacific vaccine production market is poised to register the fastest CAGR of 12.4% from 2025 to 2032, driven by rapid urbanization, expanding healthcare infrastructure, and rising demand for affordable vaccines in countries such as China, India, and Japan. Government initiatives promoting immunization, local production incentives, and increased exports of vaccines to developing countries are supporting market growth. The region is also becoming a strategic location for contract manufacturing and clinical trials.
Japan Vaccine Production Market Insight
The Japan vaccine production market is expanding due to its advanced biotechnology sector and strong national healthcare system. The country places a high priority on pandemic preparedness and aging population needs, which drives innovation in vaccine formulations. The market is expected to grow at a CAGR of 9.2% from 2025 to 2032, supported by integration of digital health platforms and increased R&D funding in cancer and infectious disease vaccines.
China Vaccine Production Market Insight
The China vaccine production market accounted for the largest revenue share in Asia-Pacific in 2024, attributed to its massive population, strong government support, and expanding biopharma capabilities. The market is expected to grow at a CAGR of 16.1% from 2025 to 2032, fueled by rapid technological adoption, government-driven immunization campaigns, and investments in both domestic production and global vaccine exports.
Vaccine Production Market Share
The vaccine production industry is primarily led by well-established companies, including:
- Sanofi (France)
- Valneva SE (France)
- CSL (Australia)
- GSK plc (U.K)
- MassBiologics (U.S)
- Johnson & Johnson Services, Inc. (U.S)
- AstraZeneca (U.K)
- Pfizer Inc. (U.S)
- Merck KGaA (Germany)
- Novartis AG (Switzerland)
- BAXTER (U.S)
- Teva Pharmaceutical Industries Ltd. (Israel)
- IDT Biologika (U.S)
- Thermo Fisher Scientific Inc. (U.S)
- Lonza (Switzerland)
- Boehringer Ingelheim International GmbH (Germany)
- Catalent, Inc (U.S)
- Charles River Laboratories (U.S)
Latest Developments in Global Vaccine Production Market
- In August 2023, Pfizer Inc. (U.S.) received U.S. FDA approval for ABRYSVO, its bivalent RSV prefusion F (RSVpreF) vaccine, for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant individuals at 32 through 36 weeks gestational age. This significant approval marked a new era in maternal immunization and is expected to contribute to the adult vaccine segment, which accounted for a dominant share of the vaccines market in 2023
- In May 2023, GSK plc (U.K.) received U.S. FDA approval for Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older. This was the first RSV vaccine approved for older adults, addressing a major unmet medical need and is set to significantly impact the adult vaccine market
- In October 2023, Lonza (Switzerland) announced an expanded collaboration with Vaxcyte for the global commercial manufacturing of broad-spectrum pneumococcal conjugate vaccines. This highlights Lonza's role as a key CDMO (Contract Development and Manufacturing Organization) in the vaccine market, supporting the production of crucial vaccines. The CDMO market segment is experiencing growth as more companies outsource complex manufacturing
- In January 2023, Sanofi (France), as part of its ongoing R&D efforts, continued to progress its vaccine pipeline. While specific product launches with percentages in January 2023 are not widely reported for the "Vaccine Production" aspect, Sanofi's commitment to vaccine innovation remains strong, with developments in areas such as influenza and RSV. For instance, Sanofi, in collaboration with AstraZeneca, later in 2023 (and accelerated in 2024 for 2025 season) significantly increased production capacity for Beyfortus (nirsevimab), an RSV immunization for infants, underscoring their dedication to meeting growing demand in key disease areas
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.