Global Uterine Fibroid Embolization Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
5.70 Billion
USD
8.05 Billion
2024
2032
USD
5.70 Billion
USD
8.05 Billion
2024
2032
| 2025 –2032 | |
| USD 5.70 Billion | |
| USD 8.05 Billion | |
|
|
|
|
Uterine Fibroid Embolization Devices Market Analysis
In recent years, Uterine fibroid embolization (UFE) have been highly deployed globally due to its minimally invasive technique. The technique incorporates the use of a real-time x-ray called fluoroscopy to guide the delivery of embolic agents to the uterus and fibroids. About 90% of women who have undergone UFE experience significant or complete resolution of their fibroid-related symptoms.
Uterine Fibroid Embolization Devices Market Size
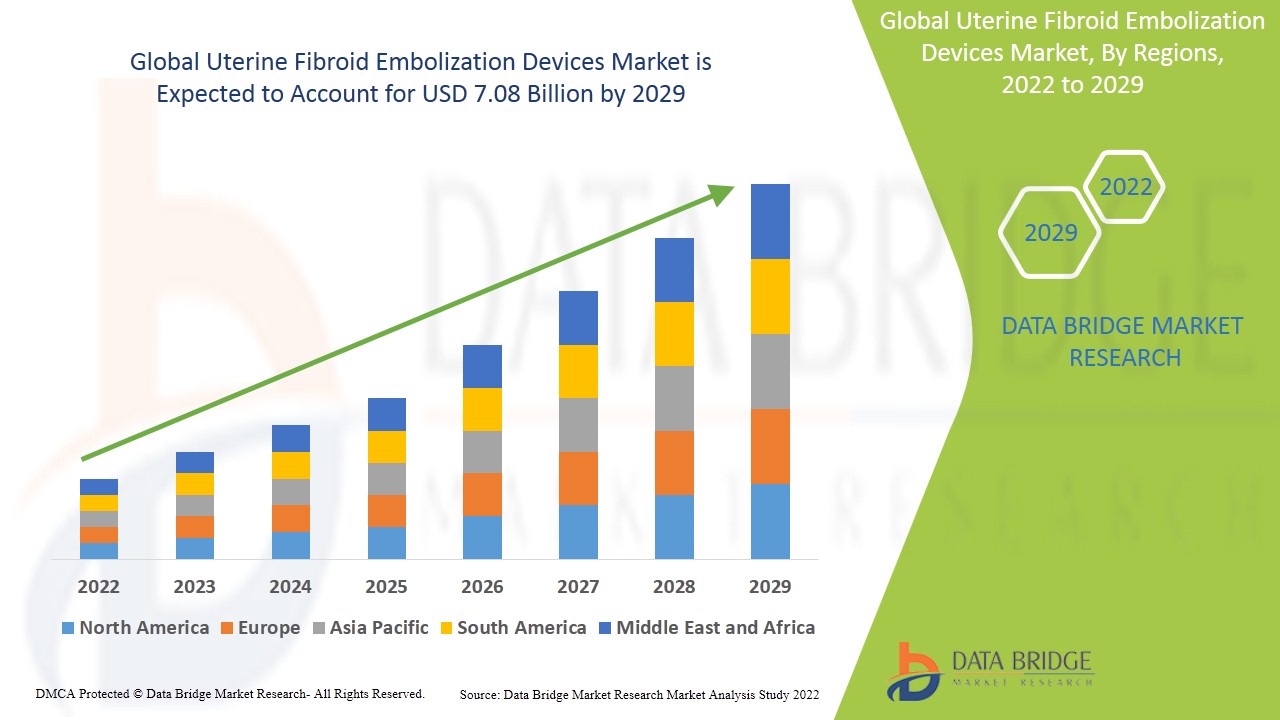
Global uterine fibroid embolization devices market size was valued at USD 5.70 billion in 2024 and is projected to reach USD 8.05 billion by 2032, with a CAGR of 4.40% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Uterine Fibroid Embolization Devices Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Boston Scientific Corporation (US), Merit Medical Systems (US), BioSpace (US), Hologic, Inc. (US), Cook (UK), Ferring B.V. (Switzerland), Smith+Nephew (UK), CooperSurgical, Inc. (US), Aetna Better Health of Pennsylvania (US), Ethicon US, LLC (US), Olympus America (US), Stryker (US), among others |
|
Market Opportunities |
|
Uterine Fibroid Embolization Devices Market Definition
Uterine fibroid embolization (UFE) refers to a minimally invasive treatment that is widely utilized for treating fibroid tumors in the uterus. The procedure is referred as a uterine artery embolization (UAE), and is used for treating other complications such as placenta previa, arteriovenous malformations of the pelvis and placenta accrete.
Uterine Fibroid Embolization Devices Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints and challenges. All of this is discussed in detail as below:
- Prevalence of Uterine Fibroids
The rise in the prevalence of uterine fibroids among women across the acts as one of the major factors driving the growth of uterine fibroid embolization devices market.
- Preference for Minimally Invasive Surgery
The increase in the preference for minimally invasive surgery owing to the advantages, such as smaller incisions decrease post-operative pain and speedy recovery, accelerate the market growth.
- Increase in Funds
The surge in funding from private and government organizations for development of healthcare sector and research centers further influence the market.
Opportunities
Furthermore, advancements in the procedure and devices devices extend profitable opportunities to the market players in the forecast period of 2025 to 2032. Also, rise in healthcare awareness among the patients will further expand the market.
Restraints/Challenges
On the other hand, high cost of medical devices and ongoing healthcare reforms are expected to obstruct market growth. Also, stringent regulations are projected to challenge the uterine fibroid embolization devices market in the forecast period of 2025-2032.
This uterine fibroid embolization devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on uterine fibroid embolization devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Uterine Fibroid Embolization Devices Market Scope
The uterine fibroid embolization devices market is segmented on the basis of technology and mode of treatment. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Technology
- Surgical Techniques
- Laparoscopic Techniques
- Ablation Techniques
- Embolization Techniques
Mode of Treatment
- Invasive Treatment
- Minimally Invasive Treatment
- Non-Invasive Treatment
Uterine Fibroid Embolization Devices Market Regional Analysis
The uterine fibroid embolization devices market is analyzed and market size insights and trends are provided by country, technology and mode of treatment as referenced above.
The countries covered in the uterine fibroid embolization devices market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the uterine fibroid embolization devices market because of the presence of well-established healthcare facilities within the region.
Asia-Pacific (APAC) is expected to witness significant growth during the forecast period of 2025 to 2032 due to the large patient base and unmet needs regarding the fibroids diagnosis and treatment in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Uterine Fibroid Embolization Devices Market Share
The uterine fibroid embolization devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to uterine fibroid embolization devices market.
Uterine Fibroid Embolization Devices Market Leaders Operating in the Market Are:
- Boston Scientific Corporation (US)
- Merit Medical Systems (US)
- BioSpace (US)
- Hologic, Inc. (US)
- Cook (UK)
- Ferring B.V. (Switzerland)
- Smith+Nephew (UK)
- CooperSurgical, Inc. (US)
- Aetna Better Health of Pennsylvania (US)
- Ethicon US, LLC (US)
- Olympus America (US)
- Stryker (US)
Latest Developments in Uterine Fibroid Embolization Devices Market
- Karl Storz has launched Tele Pack+, a compact endoscopy system in March’2020. The system is a transportable video system that assists in merging all of the key components, such as light source, display and camera control unit together required for diagnosis and treatment.
- Gynesonics, developed Sonata System in July’2020. The system is transcervical fibroid ablation system that makes use of radiofrequency energy for treating symptomatic uterine fibroids. The system detects and then target a single fibroid with a combination of radiofrequency and ultrasound.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.