Global Transcatheter Pulmonary Valve Market
Market Size in USD Million
CAGR :
% 
 USD
73.97 Million
USD
118.96 Million
2024
2032
USD
73.97 Million
USD
118.96 Million
2024
2032
| 2025 –2032 | |
| USD 73.97 Million | |
| USD 118.96 Million | |
|
|
|
|
Transcatheter Pulmonary Valve Market Analysis
The global transcatheter pulmonary valve market has witnessed significant growth in recent years, driven by advancements in minimally invasive cardiovascular procedures. Transcatheter pulmonary valve are used to treat patients with pulmonary valve diseases, especially those with congenital heart defects or post-surgical pulmonary valve dysfunction. The market's expansion is primarily attributed to the increasing prevalence of heart-related conditions and the growing preference for less invasive treatment options.
Market players are focusing on improving the design and efficiency of transcatheter pulmonary valve to enhance patient outcomes. As a result, there is a rise in the number of innovative products available, offering various solutions for different patient needs. Several leading companies have developed valves with enhanced durability, biocompatibility, and ease of implantation, which have contributed to the growing adoption of these devices.
Technological advancements in imaging and device delivery systems have also played a significant role in making these procedures more accessible and effective. Furthermore, the rising awareness about heart health and the benefits of transcatheter procedures have boosted the demand for transcatheter pulmonary valve. The market is expected to continue growing as more hospitals adopt transcatheter pulmonary valve implantation techniques, offering a less invasive alternative to traditional open-heart surgeries. This trend, coupled with ongoing research and development, ensures a promising future for the transcatheter pulmonary valve market.
Transcatheter Pulmonary Valve Market Size
The Transcatheter Pulmonary Valve market size was valued at USD 73.97 Million in 2024 and is projected to reach USD 118.96 Million by 2032, with a CAGR of 6.12% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Transcatheter Pulmonary Valve Market Trends
“Increasing shift towards minimally invasive procedures”
The increasing shift towards minimally invasive procedures in the transcatheter pulmonary valve market is a significant trend that is transforming the treatment landscape. As healthcare professionals and patients seek less traumatic alternatives to traditional open-heart surgery, the demand for transcatheter pulmonary valve implantation has surged. Minimally invasive procedures, which involve smaller incisions and reduced risk of complications, are seen as a safer option, especially for patients who may be at higher risk due to age or other health conditions.
Transcatheter pulmonary valve procedures, which are performed through a catheter inserted into a blood vessel, avoid the need for large surgical incisions. This not only leads to shorter hospital stays but also allows for quicker recovery times, enabling patients to return to their daily activities faster. The minimally invasive nature of these procedures is particularly appealing to patients, as it reduces the overall physical and emotional stress associated with recovery from traditional surgery.
For healthcare providers, these procedures offer the benefit of fewer complications, a lower risk of infection, and reduced healthcare costs in the long term. With the continued development of better catheter-based technologies and improved valve designs, the trend towards minimally invasive options is expected to grow, further driving the adoption of transcatheter pulmonary valve implantation in clinical practice.
Report Scope and Transcatheter Pulmonary Valve Market Segmentation
|
Attributes |
Transcatheter Pulmonary Valve Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Boston Scientific Corporation (U.S.), Braile Biomédica (Brazil), Artivion, Inc. (U.S.), Edwards Lifesciences Corporation (U.S.), JenaValve (Germany), LivaNova PLC (U.K.), Venus Medtech (Hangzhou) Inc. (China), Europa Group (Italy), Colibri Heart Valve (U.S.), Xeltis (Netherlands), Direct Flow Medical, Inc. (U.S.), Abbott. (U.S.), Lepu Medical Technology(Beijing)Co., Ltd. (China),Labcor Laboratórios Ltda. (Brazil), TTK (India) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Transcatheter Pulmonary Valve Market Definition
A transcatheter pulmonary valve is a medical device used to treat pulmonary valve dysfunction, typically in patients with congenital heart defects or those who have undergone previous heart surgeries. The valve is implanted through a catheter, which is a thin, flexible tube inserted into a blood vessel, usually in the groin, and guided to the heart. Unlike traditional open-heart surgery, which requires a large incision, the transcatheter pulmonary valve procedure is minimally invasive, offering quicker recovery times and reduced risks for the patient. The device is designed to restore proper blood flow through the heart and improve valve function, providing an effective treatment option for individuals with pulmonary valve issues.
Transcatheter Pulmonary Valve Market Dynamics
Drivers
- Growing Incidence of Pulmonary Valve Diseases
The prevalence of congenital heart defects and pulmonary valve diseases is rising globally, driving demand for transcatheter pulmonary valve interventions. Many patients, especially those born with conditions such as tetralogy of Fallot, require pulmonary valve replacement as they age, either due to valve deterioration or other complications. With the continuous improvement in medical care and an increase in life expectancy for these patients, there is a growing need for less invasive treatments. This rise in demand for transcatheter pulmonary valve procedures is directly linked to the increasing number of patients requiring these interventions, propelling market growth.
- Minimally Invasive Treatment Preferences
The healthcare sector is witnessing a shift toward less invasive medical procedures, as both patients and healthcare providers increasingly prioritize quick recovery times, reduced complications, and minimal scarring. Traditional open-heart surgeries often involve long hospital stays and significant recovery time. In contrast, transcatheter pulmonary valve implantation, which uses a catheter to place the valve, is associated with shorter hospital stays, faster recovery, and fewer complications, making it a preferred choice. As more patients seek out these benefits, the demand for minimally invasive procedures continues to grow, further boosting market expansion.
Opportunities
- Technological Advancements in Valve Design
Manufacturers are continuously innovating to improve the performance of transcatheter pulmonary valves. Advances in materials and designs aim to enhance the durability, biocompatibility, and flexibility of these devices. With innovations such as better valve durability and custom designs that accommodate different patient anatomies, the market can expect wider adoption and improved patient outcomes. These innovations also help address concerns about long-term valve performance, driving the market towards new growth opportunities.
- Expanding Application in Pediatric Populations
Transcatheter pulmonary valve implantation is becoming an increasingly viable option for pediatric patients, especially those with congenital heart defects. In many cases, these patients need valve replacement early in life, and traditional surgeries can present high risks. The ability to perform these procedures using a minimally invasive approach offers a significant advantage, reducing complications and improving long-term care. As more children and young adults are treated using these valves, this growing application in the pediatric demographic opens up new market opportunities.
Restraints/Challenges
- High Procedure Costs
Despite the advantages of transcatheter pulmonary valve implantation, the procedure remains costly, limiting its accessibility. The high cost of the valve, specialized equipment, and trained personnel needed for the procedure can make it prohibitive, particularly in lower-income regions or for patients without adequate insurance coverage. The financial burden may restrict the widespread adoption of these treatments, preventing a larger segment of the population from benefiting from them. Addressing these cost concerns will be critical for expanding market reach.
- Long-term Efficacy and Durability
One of the biggest challenges facing the transcatheter pulmonary valve market is ensuring the long-term effectiveness and durability of these devices. While they perform well initially, there are concerns about the longevity of these valves, particularly for younger patients who may require multiple interventions over their lifetimes. The potential need for re-operations or valve replacements as patients age is a significant concern, limiting the overall sustainability of this treatment option. Manufacturers must continue to focus on enhancing the long-term functionality of these valves to build greater confidence among healthcare providers and patients.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Transcatheter Pulmonary Valve Market Scope
The market is segmented on the basis of application, technology, raw material, and end user growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Application
- Cardiac Anomaly
- Pulmonary Atresia
- Pulmonary Stenosis
- Pulmonary Regurgitation
- Tetralogy of Fallot
- Truncus Arteriosus
- Others
Technology
- Balloon-Expanded
- Self-Expanded
Raw Materials
- Synthetic
- Tissue Engineered
End User
- Adult
- Pediatric
Transcatheter Pulmonary Valve Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, application, technology, raw material, and end user as referenced above.
The countries covered in the market report are U.S., Canada, Mexico in North America, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), Brazil, Argentina, Rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA).
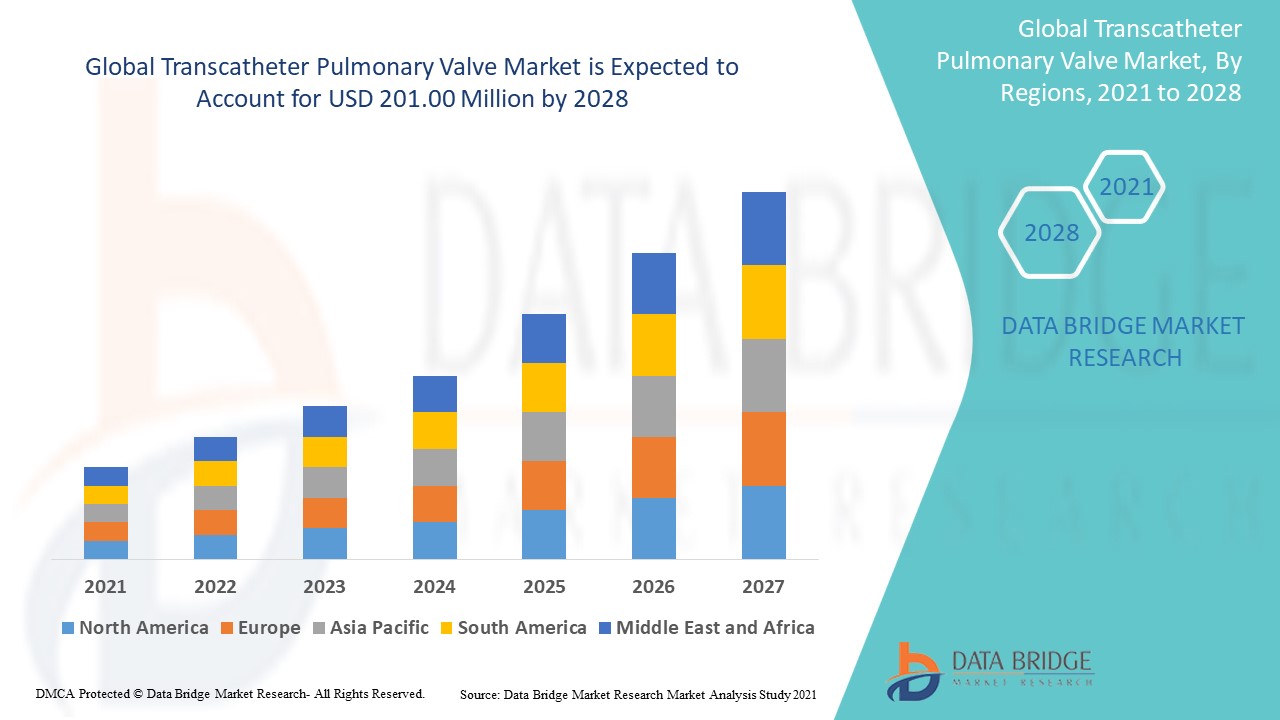
North America is currently the dominating market for transcatheter pulmonary valves, particularly the United States, the transcatheter pulmonary valve market benefits from advanced healthcare infrastructure, high healthcare expenditure, and the growing prevalence of congenital heart diseases. The availability of state-of-the-art medical facilities, skilled professionals, and a strong focus on minimally invasive procedures has propelled the region's dominance. Additionally, the increasing acceptance of transcatheter valve procedures over traditional surgeries and the presence of leading manufacturers in the region have further solidified North America’s position as the market leader.
On the other hand, the Asia Pacific region is emerging as the fastest growing market for transcatheter pulmonary valves. This growth can be attributed to the rising incidence of congenital heart defects, the expansion of healthcare infrastructure, and improving access to advanced medical treatments. Countries such as China, India, and Japan are experiencing a surge in demand for advanced cardiac care, and the growing number of middle-class populations with rising healthcare affordability is further contributing to market growth. The increasing adoption of minimally invasive heart procedures and greater awareness of treatment options are also driving the rapid growth of the transcatheter pulmonary valve market in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Transcatheter Pulmonary Valve Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Transcatheter Pulmonary Valve Market Leaders Operating in the Market Are:
- Boston Scientific Corporation (U.S.)
- Braile Biomédica (Brazil)
- Artivion, Inc. (U.S.)
- Edwards Lifesciences Corporation (U.S.)
- JenaValve (Germany)
- LivaNova PLC (U.K.)
- Venus Medtech (Hangzhou) Inc. (China)
- Europa Group (Italy)
- Colibri Heart Valve (U.S.)
- Xeltis (Netherlands)
- Direct Flow Medical, Inc. (U.S.)
- Abbott. (U.S.)
- Lepu Medical Technology(Beijing)Co., Ltd. (China)Labcor Laboratórios Ltda. (Brazil)
- TTK (India)
Latest Developments in Transcatheter Pulmonary Valve Market
- In February 2023, Medtronic announced the relaunch of its first-of-its-kind transcatheter pulmonary valve replacement system, designed for patients with congenital heart disease. This minimally invasive procedure replaces the pulmonary valve using a catheter, offering an alternative to traditional open-heart surgery. The system reduces recovery time, lowers the risk of complications, and shortens hospital stays. It is especially beneficial for patients who previously underwent heart surgery and now require a pulmonary valve replacement. The relaunch aims to improve long-term patient outcomes, enhance quality of life, and provide a less invasive, more accessible solution for those with congenital heart conditions.
- In September 2024, Edwards Lifesciences announced the launch of the SAPIEN 3 valve with the Alterra Present in Europe for transcatheter pulmonic valve implantation. This ground-breaking solution is designed to treat patients with pulmonary valve dysfunction, particularly those with congenital heart disease. The system combines the SAPIEN 3 valve, known for its durability and performance, with the Alterra Present, which provides a scaffold to stabilize the valve. This new combination enhances procedural success, reduces the risk of complications, and improves long-term patient outcomes. The innovation aims to offer a safer, minimally invasive treatment option, reducing recovery time and hospital stays for patients.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.