1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY PROCEDURE TYPE
16.1 OVERVIEW
16.2 CONVENTIONAL TACE (CTACE)
16.3 DRUG-ELUTING BEAD TACE (DEB-TACE)
16.3.1 BIODEGRADABLE BEADS
16.3.2 NON-BIODEGRADABLE BEADS
16.4 ROBOTIC-ASSISTED TACE
16.4.1 FULLY AUTONOMOUS SYSTEMS
16.4.2 SEMI-AUTONOMOUS SYSTEMS
17 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY COMPONENT
17.1 OVERVIEW
17.2 CATHETERS
17.2.1 MICROCATHETERS
17.2.2 ANGIOGRAPHIC CATHETERS
17.3 GUIDEWIRES
17.4 EMBOLIC AGENTS
17.4.1 BEADS
17.4.1.1. DRUG-ELUTING
17.4.1.2. MICROSPHERES
17.4.2 LIPIODOL
17.5 ROBOTIC SYSTEMS
17.5.1 HARDWARE
17.5.2 ROBOTIC ARMS
17.5.3 CONSOLE
17.5.4 SOFTWARE
18 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY TECHNOLOGY
18.1 OVERVIEW
18.2 MANUAL TECHNIQUES
18.3 IMAGE-GUIDED TECHNIQUES
18.3.1 DIGITAL SUBTRACTION ANGIOGRAPHY (DSA)
18.3.2 CT-GUIDED
18.3.3 MRI-GUIDED
18.4 ROBOTIC SYSTEMS
18.4.1 AI-INTEGRATED ROBOTICS
18.4.2 FLUOROSCOPY-GUIDED ROBOTICS
19 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 PRIMARY LIVER CANCER
19.3 METASTATIC LIVER CANCER
19.4 OTHER TUMORS
20 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY END-USER
20.1 OVERVIEW
20.2 HOSPITALS
20.2.1 SPECIALTY CANCER HOSPITALS
20.2.2 MULTI-SPECIALTY HOSPITALS
20.3 AMBULATORY SURGICAL CENTERS (ASCS)
20.4 CANCER RESEARCH CENTERS
21 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT SALES
21.3 DISTRIBUTORS
21.4 ONLINE PLATFORMS
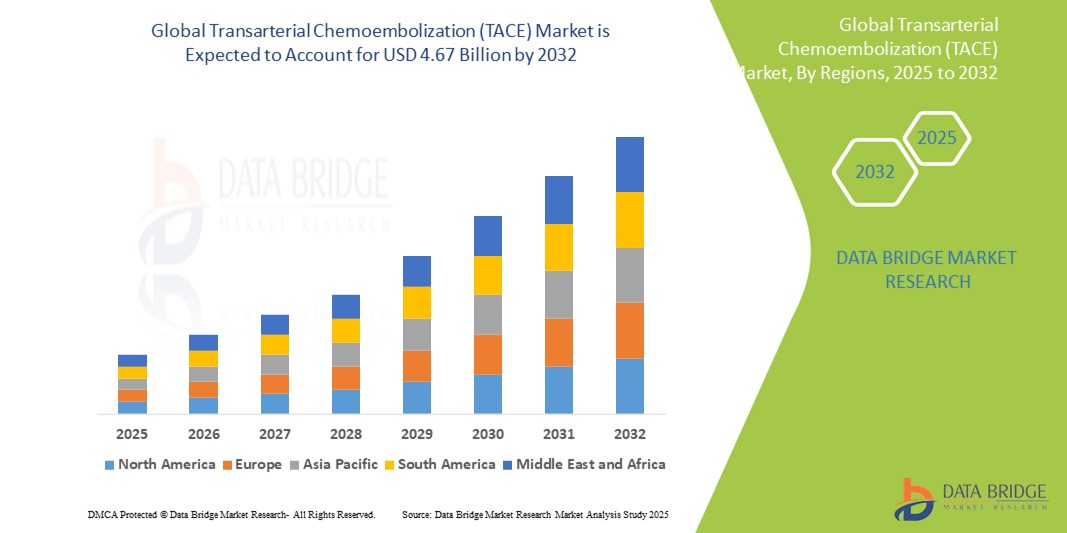
22 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, BY COUNTRY
GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.1 NORTH AMERICA
22.1.1 U.S.
22.1.2 CANADA
22.1.3 MEXICO
22.2 EUROPE
22.2.1 GERMANY
22.2.2 FRANCE
22.2.3 U.K.
22.2.4 ITALY
22.2.5 SPAIN
22.2.6 AUSTRIA
22.2.7 IRELAND
22.2.8 NORWAY
22.2.9 POLAND
22.2.10 RUSSIA
22.2.11 TURKEY
22.2.12 NETHERLANDS
22.2.13 SWITZERLAND
22.2.14 REST OF EUROPE
22.3 ASIA-PACIFIC
22.3.1 JAPAN
22.3.2 CHINA
22.3.3 SOUTH KOREA
22.3.4 INDIA
22.3.5 AUSTRALIA
22.3.6 SINGAPORE
22.3.7 THAILAND
22.3.8 MALAYSIA
22.3.9 INDONESIA
22.3.10 PHILIPPINES
22.3.11 VIETNAM
22.3.12 REST OF ASIA-PACIFIC
22.4 SOUTH AMERICA
22.4.1 BRAZIL
22.4.2 ARGENTINA
22.4.3 PERU
22.4.4 REST OF SOUTH AMERICA
22.5 MIDDLE EAST AND AFRICA
22.5.1 SOUTH AFRICA
22.5.2 UAE
22.5.3 EGYPT
22.5.4 KUWAIT
22.5.5 ISRAEL
22.5.6 REST OF MIDDLE EAST AND AFRICA
22.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS:GLOBAL
23.2 COMPANY SHARE ANALYSIS:NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS:EUROPE
23.4 COMPANY SHARE ANALYSIS:ASIA PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, SWOT AND DBR ANALYSIS
25 GLOBAL TRANSARTERIAL CHEMOEMBOLIZATION (TACE) MARKET, COMPANY PROFILE
25.1 BOSTON SCIENTIFIC CORPORATION
25.1.1 COMPANY OVERVIEW
25.1.2 KEY DECISION MAKING
25.1.3 REVENUE ANALYSIS
25.1.4 GEOGRAPHIC PRESENCE
25.1.5 PRODUCT PORTFOLIO
25.1.6 RECENT DEVELOPMENTS
25.2 MEDTRONIC PLC
25.2.1 COMPANY OVERVIEW
25.2.2 KEY DECISION MAKING
25.2.3 REVENUE ANALYSIS
25.2.4 GEOGRAPHIC PRESENCE
25.2.5 PRODUCT PORTFOLIO
25.2.6 RECENT DEVELOPMENTS
25.3 TERUMO CORPORATION
25.3.1 COMPANY OVERVIEW
25.3.2 KEY DECISION MAKING
25.3.3 REVENUE ANALYSIS
25.3.4 GEOGRAPHIC PRESENCE
25.3.5 PRODUCT PORTFOLIO
25.3.6 RECENT DEVELOPMENTS
25.4 MERIT MEDICAL SYSTEMS, INC.
25.4.1 COMPANY OVERVIEW
25.4.2 KEY DECISION MAKING
25.4.3 REVENUE ANALYSIS
25.4.4 GEOGRAPHIC PRESENCE
25.4.5 PRODUCT PORTFOLIO
25.4.6 RECENT DEVELOPMENTS
25.5 SIRTEX MEDICAL LIMITED
25.5.1 COMPANY OVERVIEW
25.5.2 KEY DECISION MAKING
25.5.3 REVENUE ANALYSIS
25.5.4 GEOGRAPHIC PRESENCE
25.5.5 PRODUCT PORTFOLIO
25.5.6 RECENT DEVELOPMENTS
25.6 COOK MEDICAL
25.6.1 COMPANY OVERVIEW
25.6.2 KEY DECISION MAKING
25.6.3 REVENUE ANALYSIS
25.6.4 GEOGRAPHIC PRESENCE
25.6.5 PRODUCT PORTFOLIO
25.6.6 RECENT DEVELOPMENTS
25.7 SIEMENS HEALTHINEERS
25.7.1 COMPANY OVERVIEW
25.7.2 KEY DECISION MAKING
25.7.3 REVENUE ANALYSIS
25.7.4 GEOGRAPHIC PRESENCE
25.7.5 PRODUCT PORTFOLIO
25.7.6 RECENT DEVELOPMENTS
25.8 GE HEALTHCARE
25.8.1 COMPANY OVERVIEW
25.8.2 KEY DECISION MAKING
25.8.3 REVENUE ANALYSIS
25.8.4 GEOGRAPHIC PRESENCE
25.8.5 PRODUCT PORTFOLIO
25.8.6 RECENT DEVELOPMENTS
25.9 PHILIPS
25.9.1 COMPANY OVERVIEW
25.9.2 KEY DECISION MAKING
25.9.3 REVENUE ANALYSIS
25.9.4 GEOGRAPHIC PRESENCE
25.9.5 PRODUCT PORTFOLIO
25.9.6 RECENT DEVELOPMENTS
25.1 STRYKER CORPORATION
25.10.1 COMPANY OVERVIEW
25.10.2 KEY DECISION MAKING
25.10.3 REVENUE ANALYSIS
25.10.4 GEOGRAPHIC PRESENCE
25.10.5 PRODUCT PORTFOLIO
25.10.6 RECENT DEVELOPMENTS
25.11 GUERBET GROUP
25.11.1 COMPANY OVERVIEW
25.11.2 KEY DECISION MAKING
25.11.3 REVENUE ANALYSIS
25.11.4 GEOGRAPHIC PRESENCE
25.11.5 PRODUCT PORTFOLIO
25.11.6 RECENT DEVELOPMENTS
25.12 CANON MEDICAL SYSTEMS CORPORATION
25.12.1 COMPANY OVERVIEW
25.12.2 KEY DECISION MAKING
25.12.3 REVENUE ANALYSIS
25.12.4 GEOGRAPHIC PRESENCE
25.12.5 PRODUCT PORTFOLIO
25.12.6 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST RELATED REPORTS
26 CONCLUSION
27 QUESTIONNAIRE
28 ABOUT DATA BRIDGE MARKET RESEARCH