Global Third Generation Ventricular Assist Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
2.50 Billion
USD
9.08 Billion
2022
2030
USD
2.50 Billion
USD
9.08 Billion
2022
2030
| 2023 –2030 | |
| USD 2.50 Billion | |
| USD 9.08 Billion | |
|
|
|
|
Third Generation Ventricular Assist Devices Market Analysis and Size
According to the World Health Organization 2021, an estimated 17.9 million people die each year as a result of cardiovascular diseases worldwide. This accounts for 35% of all deaths worldwide. Furthermore, heart attacks and stroke account for 85% of all cardiovascular disease deaths. According to a July 2020 article published in Cureus Journal of Medical Science, ischemic heart disease (IHD) is a leading cause of death worldwide. Ischemic heart disease affects approximately 126 million people (1,655 per 100,000), or about 1.72% of the world's population. By 2030, the global prevalence of ischemic heart disease is expected to exceed 1,845 per 100,000 people.
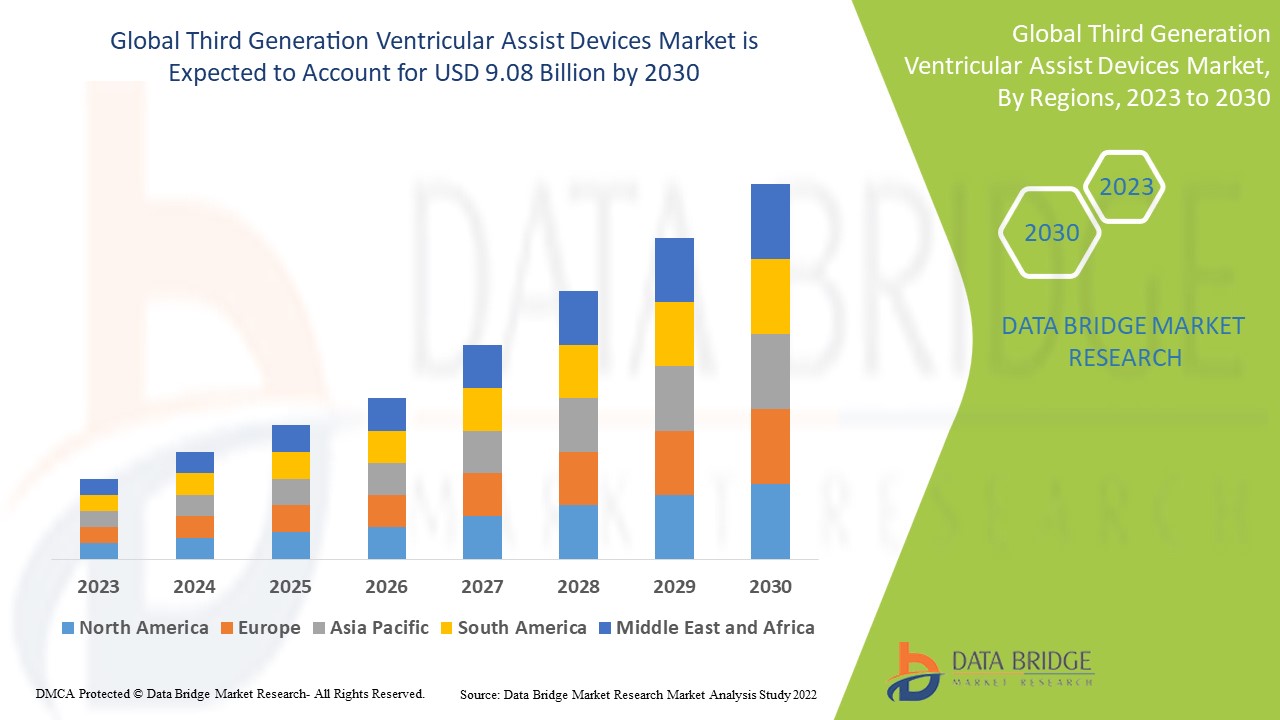
Data Bridge Market Research analyses that the third generation ventricular assist devices market which was USD 2.5 billion in 2022, is expected to reach USD 9.08 billion by 2030, at a CAGR of 17.50% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Third Generation Ventricular Assist Devices Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Left Ventricular Assist Devices (LVADs), Right Ventricular Assist Devices (RVADs), Biventricular Assist Devices (BIVADs), Percutaneous Ventricular Assist Devices (PVADs), Total Artificial Heart (TAH)), Application (Destination Therapy, Bridge-to-Candidacy (BTC) Therapy, Bridge-to-Transplant (BTT) Therapy, Bridge-to-Recovery (BTR) Therapy, Others), Age (Below 18 Years, 19-39 Years, 40-59 Years, 60-79 Years, Above 80 Years), Flow (Pulsatile Flow, Continuous Flow), Design (Transcutaneous, Implantable), Technology (Magnetic, Hydrodynamic Levitation) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
ABIOMED (U.S.), Asahi Kasei Corporation (Japan), Abbott (U.S.), Calon Cardio (U.K.), Jarvik Heart, Inc. (U.S.), Medtronic (Ireland), Terumo Corporation (Japan), BiVACOR Inc. (U.S.), Cardiobridge GmbH (Germany), CHF Solutions, Inc. (U.S.), Evaheart, Inc. (U.S.), LivaNova PLC (U.K.), CorWave SA (France), Fresenius Medical Care AG & Co. KGaA (Germany), MiTiHeart Corporation (U.S.), Getinge AB (Sweden), SynCardia Systems, LLC (U.S.), Teleflex Incorporated (U.S.), Mayo Foundation for Medical Education and Research (MFMER) (U.S.) |
|
Market Opportunities |
|
Market Definition
Third-generation ventricular assist devices (VADs) are continuous-flow rotary pumps with a fully levitated impeller/rotor and no mechanical contact between the rotating and stationary parts of the pump in normal operation. Third-generation VADs have emerged with the promise of improved hemocompatibility and durability over second-generation devices. However, most third-generation devices have not demonstrated a significant advantage over their second-generation counterparts, frequently trading an improvement in one aspect of performance for a disadvantage in another.
Global Third Generation Ventricular Assist Devices Market Dynamics
Drivers
- Technological advancements
The VAD market is constantly changing, with key players investing in product innovation and development. The market for VAD is expected to be driven by technological advancements combined with timely regulatory approvals in the form of small-size devices with features such as infection control. Berlin Heart announced in June 2020 that the US FDA's required Post Market Approval (PMA) for EXCOR Pediatric VAD was completed. Furthermore, benefits associated with the use of VADs include increased abidance, functionality, and durability, which increase product adoption and thus support market growth.
- Rising private equity firms
Private equity firms and venture capitalists are making investments in the growing third generation VAD market to promote product development. For instance, CorWave raised USD 40.0 million in a Series C funding round led by three new and returning investors in January 2021 for its implantable heart pump based on a novel technology, the wave membrane pump, including Novo Holdings, Bpifrance, Sofinnova Partners, Seventure, Ysios Capital, European Commission, Singaporean family office M&L Healthcare, and Financière Arbevel.
Opportunities
- Novel advancements
Advances in novel VADs for surgical interventions, particularly in paediatric patients suffering from cardiac disorders, are expected to increase the demand. For instance, the US Food and Drug Administration graded the continuous-flow LVADs, Micromed HeartAssist 5TM Pediatric VAD, and Berlin HeartTM EXCOR paediatric VAD for paediatric patients. Since 2011, Berlin HeartTM EXCOR has been approved for use as a bridge-to-transplantation device.
•Increasing incidence of organ transplantation
The rising prevalence of cardiovascular diseases and growing risks of organ failures is supporting the growth in organ transplant demand. The shortage of organ donors delays the organ transplantation procedure. As per United Network for Organ Sharing, nearly 121,016 people need organ transplantation and approximately 77,725 people are on the active waiting list.
Restraints/Challenges
- High costs
High costs associated with procedure and device usage, a lack of government reimbursement policies, and an increasing number of serious risks involved in procedure are acting as market restraints for the growth of third-generation ventricular assist devices during the forecast period. The most difficult challenge for market growth will be the increased development of affordable, small, and efficient devices.
This third generation ventricular assist devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the third generation ventricular assist devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Third Generation Ventricular Assist Devices Market
The ongoing Covid-19 pandemic has had a negative impact on the third generation ventricular assist devices market, with the government imposing temporary lockdowns and travel restrictions, resulting in a decrease in the number of surgical procedures. For instance, the global revenue of the ABIOMED Impella heart pump was impacted in the fourth quarter of FY2020 due to lower patient utilization and the significant impact of the COVID-19 pandemic on surgeries, elective medical procedures, and fewer patients seeking treatment at hospitals.
Recent Developments
- In June 2020, The FDA approved the company's First-in-Human Trial of Impella ECP, which will be studied in high-risk percutaneous coronary intervention patients. In addition, to strengthen their business and expand the reach of their device, they are implementing various strategies such as mergers and acquisitions and expanding their manufacturing facilities.
Global Third Generation Ventricular Assist Devices Market Scope
The third generation ventricular assist devices market is segmented on the basis of product, application, age, flow, design, and technology. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Left Ventricular Assist Devices (LVADs)
- Right Ventricular Assist Devices (RVADs)
- Biventricular Assist Devices (BIVADs)
- Percutaneous Ventricular Assist Devices (PVADs)
- Total Artificial Heart (TAH)
Application
- Destination Therapy
- Bridge-to-Candidacy (BTC) Therapy
- Bridge-to-Transplant (BTT) Therapy
- Bridge-to-Recovery (BTR) Therapy
- Others
Age
- Below 18 Years
- 19-39 Years
- 40-59 Years
- 60-79 Years
- Above 80 Years
Flow
- Pulsatile Flow
- Continuous Flow
- Axial continuous flow
- Centrifugal continuous flow
Design
- Transcutaneous
- Implantable
Technology
- Magnetic
- Hydrodynamic Levitation
Third Generation Ventricular Assist Devices Market Regional Analysis/Insights
The third generation ventricular assist devices market is analyzed and market size insights and trends are provided by country, product, application, age, flow, design, and technology as referenced above.
The countries covered in the third generation ventricular assist devices market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the third generation ventricular assist devices market because of rising public health awareness, the prevalence of well-established healthcare policies, and the region's high prevalence of cardiovascular disorders.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 due to the growing number of initiatives such as “Make in India” along with surging levels of investment for the growth of the healthcare sector in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The third generation ventricular assist devices market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for third generation ventricular assist devices market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the third generation ventricular assist devices market. The data is available for historic period 2011-20201.
Competitive Landscape and Third Generation Ventricular Assist Devices Market Share Analysis
The third generation ventricular assist devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to third generation ventricular assist devices market.
Some of the major players operating in the third generation ventricular assist devices market are:
- ABIOMED (U.S.)
- Asahi Kasei Corporation (Japan)
- Abbott (U.S.)
- Calon Cardio (U.K.)
- Jarvik Heart, Inc. (U.S.)
- Medtronic (Ireland)
- Terumo Corporation (Japan)
- BiVACOR Inc. (U.S.)
- Cardiobridge GmbH (Germany)
- CHF Solutions, Inc. (U.S.)
- Evaheart, Inc. (U.S.)
- LivaNova PLC (U.K.)
- CorWave SA (France)
- Fresenius Medical Care AG & Co. KGaA (Germany)
- MiTiHeart Corporation (U.S.)
- Getinge AB (Sweden)
- SynCardia Systems, LLC (U.S.)
- Teleflex Incorporated (U.S.)
- Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
Research Methodology: Global Third Generation Ventricular Assist Devices Market
Data collection and base year analysis is done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. Also market share analysis and key trend analysis are the major success factors in the market report. To know more please request an analyst call or can drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Factbook) or can assist you in creating presentations from the data sets available in the report.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












