Global Subdermal Contraceptive Implants Market Segmentation, By Type (Etonogestrel Implant, Levonorgestrel Implant, and Others), Application (Hospital, Clinic, and Research Facilities) – Industry Trends and Forecast to 2031
Subdermal Contraceptive Implants Market Analysis
The subdermal contraceptive implants market is experiencing notable growth due to increasing demand for long-term, reversible contraceptive methods among women. These implants, which provide effective pregnancy prevention for up to three to five years, offer a convenient alternative to traditional methods such as pills and injections. The market is driven by rising awareness of reproductive health, favorable government initiatives, and advancements in implant technology that enhance safety and efficacy. Recent developments include the introduction of innovative implant designs that minimize side effects and improve user experience. Additionally, collaborations among pharmaceutical companies and healthcare providers aim to expand access to these contraceptives, particularly in developing regions. As healthcare systems increasingly recognize the importance of family planning and women's health, the subdermal contraceptive implants market is poised for sustained growth, supported by a combination of technological advancements and increased public health initiatives focused on reproductive rights.
Subdermal Contraceptive Implants Market Size
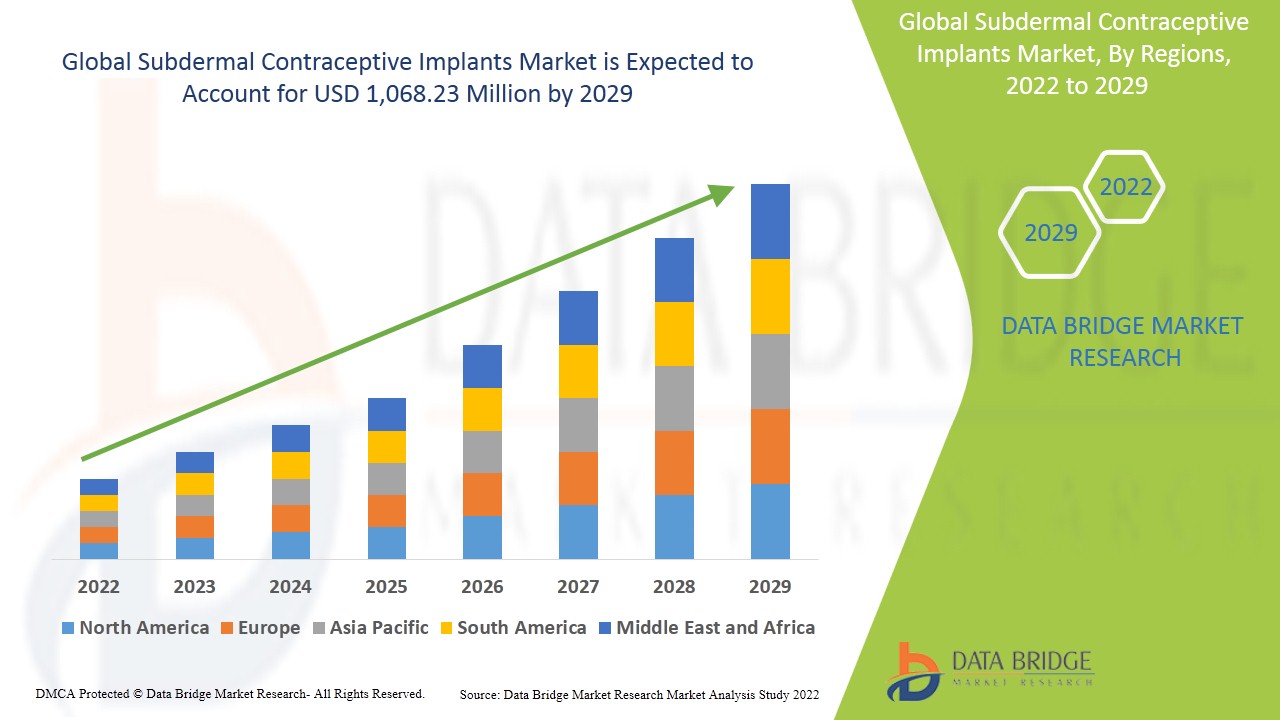
The global subdermal contraceptive implants market size was valued at USD 878.13 million in 2023 and is projected to reach USD 1140.33 million by 2031, with a CAGR of 3.32% during the forecast period of 2024 to 2031. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Subdermal Contraceptive Implants Market Trends
“Adoption of Biocompatible Materials”
The subdermal contraceptive implants market is witnessing significant trends driven by innovations in reproductive health technology. One notable trend is the increasing adoption of biocompatible materials that enhance the safety and comfort of implants, leading to improved patient satisfaction and adherence. These advancements aim to reduce side effects and improve the overall effectiveness of the implants, making them a more attractive option for long-term contraception. Additionally, the market is seeing a rise in awareness campaigns and educational initiatives that promote the benefits of subdermal implants, particularly in developing regions. As healthcare providers and policymakers recognize the importance of accessible family planning solutions, the subdermal contraceptive implants market is expected to expand, driven by both technological advancements and increased demand for effective contraceptive options.
Report Scope and Subdermal Contraceptive Implants Market Segmentation
|
Attributes
|
Subdermal Contraceptive Implants Key Market Insights
|
|
Segments Covered
|
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
|
|
Key Market Players
|
Merck & Co., Inc. (U.S.), Shanghai Dahua Pharmaceutical Co., Ltd (China), Bayer AG (Germany), Baxter (U.S.), BD (U.S.), Gerresheimer AG (Germany), Pfizer, Inc. (U.S.), Novartis AG (Switzerland), SCHOTT (Germany), Eli Lilly and Company (U.S.), Sandoz Group AG (Switzerland), Terumo Corporation (Japan), Teva Pharmaceutical Industries Ltd. (Israel), West Pharmaceutical Services, Inc. (U.S.), SHL Medical AG (Switzerland), Insulet Corporation (U.S.)
|
|
Market Opportunities
|
|
|
Value Added Data Infosets
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Subdermal Contraceptive Implants Market Definition
Subdermal contraceptive implant is a tiny, thin rod about the size of a matchstick. The implant releases hormones slow and stable into the body to prevent pregnancy. Subdermal contraceptive implant helps to prevent pregnancy for one to five years. The protection period depends upon specific progestin and the type of polymer.
Subdermal Contraceptive Implants Market Dynamics
Drivers
- Increasing Demand for Long-Term Contraceptive Options
The growing preference for long-acting reversible contraceptives (LARCs) among women significantly drives the subdermal contraceptive implants market. LARCs, which include subdermal implants, offer a highly effective means of preventing pregnancy for extended periods, typically ranging from three to five years. Unlike traditional contraceptive methods that require daily or frequent attention, LARCs provide a convenient solution for women seeking reliable birth control without the hassle of regular maintenance. This ease of use, coupled with high efficacy rates and the ability to reverse the method whenever desired, has led to increased acceptance and demand for subdermal implants, making them a preferred choice in family planning.
- Rising Healthcare Expenditure
Increased healthcare spending and enhanced access to healthcare services are crucial drivers for the growth of the subdermal contraceptive implants market, particularly in underserved populations. As governments and organizations allocate more resources to healthcare, the availability of various contraceptive options, including subdermal implants, is improving. This financial investment leads to better infrastructure, training for healthcare providers, and outreach programs that educate women about family planning. Additionally, improved access to healthcare services helps eliminate barriers, such as cost and availability, making it easier for women in low-income or rural areas to obtain these long-acting contraceptives. As a result, more women can benefit from the effectiveness and convenience of subdermal implants.
Opportunities
- Integration with Digital Health Solutions
Combining subdermal contraceptive implants with digital health platforms represents a significant market opportunity to enhance patient management and follow-up care. By integrating mobile applications that remind users about their healthcare appointments and track menstrual cycles, healthcare providers can foster greater adherence to contraceptive plans. Patient management software and services can provide users with personalized reminders, educational content, and support resources, improving their overall experience with subdermal implants. Furthermore, such platforms can facilitate communication between patients and healthcare professionals, allowing for timely interventions and adjustments as needed. As women increasingly seek tech-driven solutions for their healthcare needs, the integration of digital health platforms with subdermal implants can lead to higher user satisfaction and increased market penetration, ultimately benefiting both patients and providers.
- Technological Advancements
Continuous innovation in the design and materials used for subdermal contraceptive implants presents a valuable market opportunity to improve user experience and safety. By focusing on the development of implants that minimize side effects and extend the duration of effectiveness, manufacturers can make these contraceptives more appealing to potential users. Innovations such as biocompatible materials and advanced hormone delivery systems can enhance comfort and efficacy, ultimately leading to higher satisfaction rates among users. As women increasingly prioritize safe and effective contraceptive options, advancements in implant technology can drive greater adoption and significantly expand the market for subdermal contraceptive implants.
Restraints/Challenges
- High Initial Costs
The high cost of manufacturing and distributing subdermal contraceptive implants presents a significant challenge to their widespread accessibility, particularly for women in low-income regions. These costs encompass the production of the implants themselves and the expenses associated with distribution and healthcare services required for insertion and removal. In many underserved areas, where affordable healthcare options are already limited, the price of subdermal implants can be prohibitive. This financial barrier can deter women from considering this effective contraceptive method, leading to unmet family planning needs and continued reliance on less effective options. Addressing the cost issue is crucial to improving accessibility and ensuring that all women, regardless of their economic situation, can benefit from the advantages offered by subdermal contraceptive implants.
- Shortage of Trained Professionals
Subdermal contraceptive implants market is the limited availability of trained healthcare professionals to perform the necessary procedures for insertion and removal. In many regions, especially in developing countries, only around 30% of healthcare providers have the specialized training required for handling subdermal implants. This creates a significant barrier to access, as women in rural or underserved areas may face challenges in finding qualified professionals. The lack of widespread training further contributes to low adoption rates, making it difficult to expand the market and reach populations that would benefit from the technology.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Subdermal Contraceptive Implants Market Scope
The market is segmented on the basis of type and application. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Etonogestrel Implant
- Levonorgestrel Implant
- Others
Application
- Hospital
- Clinic
- Research Facilities
Subdermal Contraceptive Implants Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, and application as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America leads the subdermal contraceptive implants market, driven by a growing awareness of contraceptive options and enhanced education surrounding reproductive health. The region's rising disposable income also plays a crucial role, enabling individuals to invest in long-term family planning solutions. These factors contribute to the increasing acceptance and utilization of subdermal contraceptive implants among consumers in North America.
The Asia-Pacific region is projected to experience substantial growth from 2024 to 2031, primarily driven by increased government initiatives aimed at promoting reproductive health. These initiatives include awareness campaigns and enhanced access to contraceptive options, which are likely to encourage the adoption of subdermal contraceptive implants. As a result, the supportive policy environment is expected to significantly boost market expansion in this area.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Subdermal Contraceptive Implants Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Subdermal Contraceptive Implants Market Leaders Operating in the Market Are:
- Merck & Co., Inc. (U.S.)
- Shanghai Dahua Pharmaceutical Co., Ltd (China)
- Bayer AG (Germany)
- Baxter (U.S.)
- BD (U.S.)
- Gerresheimer AG (Germany)
- Pfizer, Inc. (U.S.)
- Novartis AG (Switzerland)
- SCHOTT (Germany)
- Eli Lilly and Company (U.S.)
- Sandoz Group AG (Switzerland)
- Terumo Corporation (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
- West Pharmaceutical Services, Inc. (U.S.)
- SHL Medical AG (Switzerland)
- Insulet Corporation (U.S.)
Latest Developments in Subdermal Contraceptive Implants Market
- In February 2024, Bayer AG partnered with DARÉ BIOSCIENCE to enhance the development of an innovative hormone-free monthly contraceptive option. This collaboration aims to address the growing demand for non-hormonal contraceptive methods, providing consumers with more choices in family planning. By combining their expertise, the two companies seek to advance reproductive health solutions and improve accessibility to effective contraceptive alternatives
- In February 2023, the Family Planning Association of India (FPAI) launched efforts to promote Implanon NXT, a new contraceptive option. This subdermal single-rod implant offers a long-term, reversible contraceptive solution, providing a modern alternative to older implant types such as Norplant, Jadelle, Sino-implant, and the original Implanon. By advocating for Implanon NXT, FPAI aims to enhance reproductive health choices and improve accessibility to effective family planning methods
- In April 2022, ProMed unveiled the start of preclinical evaluations for resorbable contraceptive implants, a pioneering project backed by the Bill & Melinda Gates Foundation. This initiative focuses on developing cutting-edge contraceptive options that enhance patient safety and comfort, addressing the needs of diverse populations. With the foundation's significant support, ProMed aims to drive forward research and innovation in reproductive health, potentially transforming contraceptive choices for individuals worldwide
SKU-