Global Structural Heart Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
3.06 Billion
USD
6.59 Billion
2024
2032
USD
3.06 Billion
USD
6.59 Billion
2024
2032
| 2025 –2032 | |
| USD 3.06 Billion | |
| USD 6.59 Billion | |
|
|
|
|
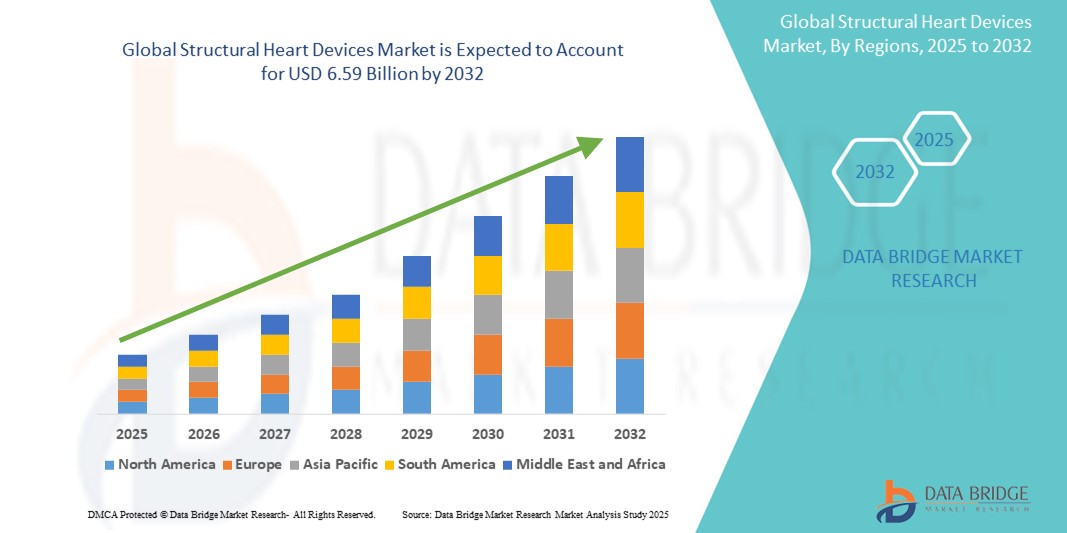
Structural Heart Devices Market Size
- The global structural heart devices market was valued at USD 3.06 billion in 2024 and is expected to reach USD 6.59 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 10.08%, primarily driven by the prevalence of cardiac disease
- This growth is driven by factors such as the aging population, growing patients awareness.
Structural Heart Devices Market Analysis
- Structural heart devices are vital tools used in treating a range of heart defects, including aortic stenosis, mitral regurgitation, and atrial septal defects, by supporting or replacing damaged heart structures through minimally invasive or surgical interventions
- The demand for these devices is being significantly driven by the increasing prevalence of structural heart diseases, especially among the elderly population, alongside rising incidences of congenital heart defects in new-borns
- North America stands out as the dominant region in the structural heart devices market due to its highly developed healthcare infrastructure, widespread adoption of cutting-edge cardiac technologies, and strong presence of key medical device manufacturers
- For instance, the U.S. has seen a rapid increase in the adoption of transcatheter aortic valve replacement (TAVR) procedures, supported by favourable reimbursement policies and ongoing clinical trials demonstrating long-term efficacy and safety
- Globally, structural heart devices are considered one of the most transformative innovations in the cardiovascular field, second only to traditional stent-based interventions, due to their role in improving survival rates and quality of life for patients with complex cardiac conditions
Report Scope and Structural Heart Devices Market Segmentation
|
Attributes |
Structural Heart Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Structural Heart Devices Market Trends
“Integration of Advanced Imaging and AI-Guided Navigation”
- One of the key emerging trends in the global structural heart devices market is the integration of advanced imaging modalities (such as 3D echocardiography, CT, and MRI) and AI-guided navigation systems to support complex structural heart interventions
- These technologies enhance the precision and safety of procedures such as transcatheter valve replacements and repairs by providing real-time, high-resolution, and dynamic imaging of cardiac structures
- For instance, 3D echocardiography allows cardiologists to visualize cardiac anatomy in greater detail, which is critical for procedures such as transcatheter mitral valve repair (TMVR) and left atrial appendage closure (LAAC)
- AI and machine learning algorithms further aid in automated image analysis, procedural planning, and risk assessment, helping clinicians make faster and more informed decisions
- This trend is revolutionizing the field of structural heart therapy, leading to better clinical outcomes, shorter procedure times, and expanding the scope of minimally invasive cardiac interventions for high-risk and elderly populations
Structural Heart Devices Market Dynamics
Driver
“Growing Prevalence of Cardiovascular Diseases”
- The growing prevalence of cardiovascular diseases (CVDs) such as coronary artery disease, heart failure, and arrhythmias is significantly contributing to the increased demand for structural heart devices
- As the global population ages and lifestyle factors such as poor diet, lack of physical activity, and smoking persist, the incidence of cardiovascular diseases continues to rise, increasing the need for advanced heart treatments and devices
- Conditions such as coronary artery disease and heart failure, in particular, are major causes of morbidity and mortality worldwide, driving the demand for devices such as heart valves, stents, and pacemakers to restore normal heart function
- Ongoing advancements in the field of cardiology, such as minimally invasive techniques and the development of novel, more durable structural heart devices, further highlight the need for cutting-edge equipment that supports better outcomes in heart disease treatments
- As more individuals are diagnosed with cardiovascular diseases and seek treatment, the demand for structural heart devices continues to grow, ensuring better surgical outcomes and reducing the risks associated with heart procedures
For instance,
- In May 2023, according to an article published by the American Heart Association, the prevalence of cardiovascular diseases among people aged 65 and older is expected to rise sharply, increasing the need for structural heart devices for managing these conditions
- In November 2022, a study published by the World Health Organization predicted that by 2030, cardiovascular diseases would become the leading cause of death worldwide, further driving the need for heart device interventions in healthcare settings
- As a result of the growing prevalence of cardiovascular diseases, advancements in minimally invasive techniques, and an aging population, the demand for structural heart devices is expected to see significant growth
Opportunity
“Advancements in Minimally Invasive Surgery and AI Integration”
- One of the key opportunities in the global structural heart devices market is the integration of artificial intelligence (AI) and advanced imaging into minimally invasive surgical procedures
- AI and real-time imaging can enhance procedural accuracy, allowing for more personalized treatments, reducing complications, and improving long-term outcomes for patients undergoing heart valve replacement or repair
- Furthermore, the development of AI-driven guidance systems can assist surgeons in selecting the most appropriate device, optimizing the surgical approach, and predicting patient-specific complications or risks
For instance,
- In January 2025, the integration of AI algorithms for valve sizing and procedure planning is expected to revolutionize the market, offering enhanced precision in patient selection for TAVR and TMVR. AI can assist in automating measurements and identifying complex anatomy that might otherwise be challenging for surgeons
- The integration of AI and advanced imaging into minimally invasive procedures offers enhanced precision, personalized treatments, and improved outcomes in the structural heart devices market
Restraint/Challenge
“High Costs and Reimbursement Issues”
- The high cost of structural heart devices remains a significant challenge in the market, limiting their adoption, particularly in emerging economies and smaller healthcare facilities with limited budgets
- Devices such as transcatheter valves and heart pumps can be extremely expensive, and the financial burden on healthcare systems and patients is substantial, especially considering the ongoing need for follow-up procedures and lifelong monitoring
- In addition to the device costs, reimbursement challenges in certain regions further limit the accessibility of these devices, particularly in regions where healthcare insurance systems do not fully cover advanced treatments or minimally invasive surgeries
For instance,
- According to a 2024 study by the European Society of Cardiology, high costs and inconsistent reimbursement policies for procedures such as TAVR and TMVR in regions such as Asia and Latin America are restricting market growth, particularly in hospitals with limited financial resources
- The high cost of structural heart devices and reimbursement challenges, especially in emerging economies, are significant barriers to market growth and adoption
Structural Heart Devices Market Scope
The market is segmented on the basis by product, procedure, age group and indication and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
By Procedure |
|
|
By Age group |
|
|
By Indication
|
|
|
By End User |
|
Structural Heart Devices Market Regional Analysis
“North America is the Dominant Region in the Structural Heart Devices Market”
- North America dominates the structural heart devices market due to its advanced healthcare infrastructure, high prevalence of cardiovascular diseases, and strong presence of key industry players
- U.S. accounts for the largest market share, driven by widespread adoption of transcatheter heart procedures such as TAVR (Transcatheter Aortic Valve Replacement) and TMVR (Transcatheter Mitral Valve Repair), supported by favourable reimbursement frameworks
- Increased R&D investments, a large pool of skilled cardiologists, and the availability of cutting-edge diagnostic and imaging technologies contribute significantly to market leadership
- In addition, the high demand for minimally invasive cardiac procedures and growing awareness regarding early diagnosis and treatment of structural heart diseases continue to propel market growth across the region
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to witness the highest growth rate in the structural heart devices market, driven by rapid improvements in healthcare infrastructure, expanding insurance coverage, and a growing aging population susceptible to valvular heart diseases
- Countries such as China, India, and Japan are emerging as key growth markets due to the rising burden of cardiovascular conditions and increasing adoption of advanced interventional cardiology techniques
- Japan leads in procedural volumes for structural heart interventions, thanks to its technologically advanced healthcare system and strong clinical research ecosystem
- Meanwhile, India and China are seeing rising procedural volumes and increased public-private investments in heart care, along with a greater focus on expanding access to minimally invasive cardiac procedures
- The growing presence of international device manufacturers and the development of training centres across Asia-Pacific further accelerate regional market growth
Structural Heart Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Edwards Lifesciences Corporation (U.S.)
- Medtronic (Ireland)
- Abbott (U.S.)
- Boston Scientific Corporation (U.S.)
- Cordis (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Terumo Corporation (Japan)
- LivaNova PLC (U.K.)
- Shockwave Medical Inc. (U.S.)
- Artivion, Inc (U.S.)
- ATRICURE, INC. (U.S.)
- Biotronik (Germany)
- Intuitive Surgical (U.S.)
- Xeltis (The Netherlands)
- Artivion, Inc (U.S.)
- Venus Medtech (Hangzhou) Inc. (China)
- Meril Life Sciences Pvt. Ltd. (India)
- JenaValve (U.S.)
- Corcym Group (Italy)
Latest Developments in Global Structural Heart Devices Market
- In February 2025, Edwards Lifesciences received expanded FDA approval for its SAPIEN 3 Ultra RESILIA transcatheter heart valve system for use in low-risk patients with severe aortic stenosis. The device features RESILIA tissue technology designed to reduce calcification and extend valve durability, supporting broader adoption in younger patient populations
- In November 2024, Abbott launched its TriClip Transcatheter Tricuspid Valve Repair System in Europe following CE Mark approval. The device provides a minimally invasive treatment for tricuspid regurgitation and builds upon the company’s MitraClip platform, representing a major innovation in treating previously underserved valve diseases
- In October 2024, Medtronic unveiled new clinical data at the TCT 2024 conference demonstrating the long-term safety and efficacy of its Evolut PRO+ TAVR system, particularly in bicuspid aortic valve patients. These findings support expanded indications and global market penetration of the platform
- In September 2024, Boston Scientific announced the commercial availability of its ACURATE neo2 Aortic Valve System in key European markets. The system is designed for improved sealing and ease of implantation, offering an alternative to current TAVR solutions and targeting a broader range of anatomical variations
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.