Global Software As A Medical Device Samd Market
Market Size in USD Billion
CAGR :
% 
 USD
1.58 Billion
USD
6.87 Billion
2024
2032
USD
1.58 Billion
USD
6.87 Billion
2024
2032
| 2025 –2032 | |
| USD 1.58 Billion | |
| USD 6.87 Billion | |
|
|
|
|
Software as a Medical Device (SaMD) Market Size
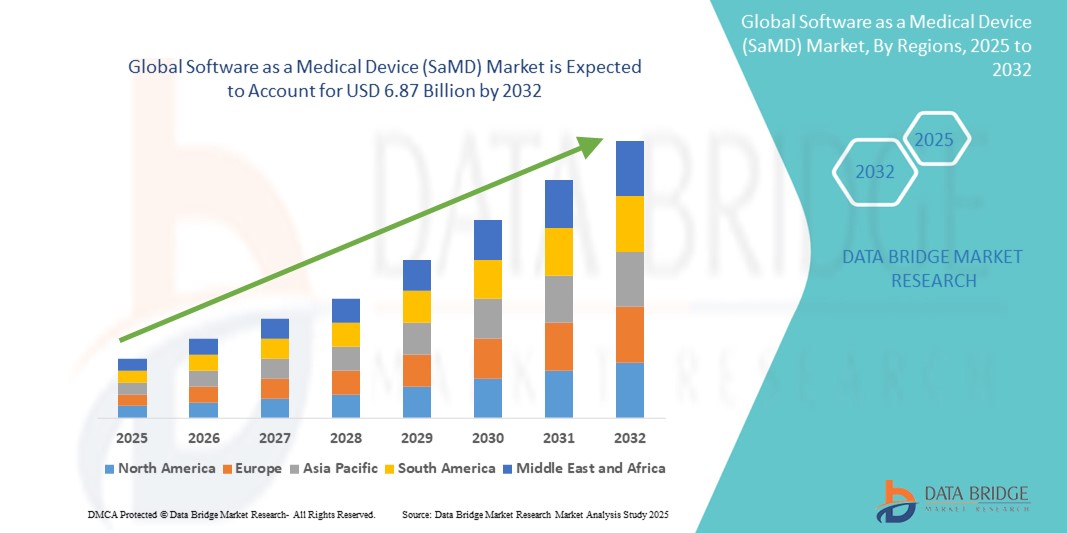
- The global software as a medical device (SaMD) market size was valued at USD 1.58 billion in 2024 and is expected to reach USD 6.87 billion by 2032, at a CAGR of 20.13% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within artificial intelligence (AI), cloud computing, and connected health platforms, leading to increased digitalization in diagnostics, monitoring, and therapeutic areas
- Furthermore, rising consumer demand for secure, user-friendly, and regulatory-compliant digital health solutions is establishing Software as a Medical Device (SaMD) as a preferred choice for modern, patient-centric care delivery. These converging factors are accelerating the uptake of software as a medical device (SaMD) solution, thereby significantly boosting the industry's growth
Software as a Medical Device (SaMD) Market Analysis
- Software as a Medical Device (SaMD), which refers to software intended for medical purposes without being part of a hardware medical device, is becoming increasingly essential in modern healthcare systems due to its capabilities in diagnostics, monitoring, and disease management across both clinical and remote settings. These solutions offer enhanced accessibility, real-time insights, and integration with digital health ecosystems
- The surging demand for SaMD is primarily driven by the global rise in chronic diseases, the proliferation of connected health platforms, favorable regulatory pathways, and the increasing need for patient-centric care solutions. Advancements in AI, cloud computing, and mobile health technologies are further accelerating adoption across healthcare providers and patients
- North America dominated the software as a medical device (SaMD) market with the largest revenue share of 44.8% in 2024, attributed to early digital health adoption, supportive regulatory frameworks from the FDA, and robust investment in health tech startups. The U.S. leads the regional market, driven by the widespread integration of AI-powered clinical decision support systems, remote patient monitoring tools, and diagnostic app
- Asia-Pacific is projected to be the fastest growing region in the software as a medical device (SaMD) market, registering a CAGR of 21.6% from 2025 to 2032, due to increasing smartphone penetration, government-led digital health initiatives, growing telehealth adoption, and rising healthcare awareness in countries such as China, India, and Japan
- The cloud-based segment dominated the software as a medical device (SaMD) market with the largest revenue share of 65.4% in 2024, driven by the increasing adoption of scalable, interoperable digital healthcare platforms and growing demand for remote patient care. Cloud-based SaMD offers real-time updates, global accessibility, and integration with telemedicine—making it the most preferred deployment model among healthcare providers
Report Scope and Software as a Medical Device (SaMD) Market Segmentation
|
Attributes |
Software as a Medical Device (SaMD) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Software as a Medical Device (SaMD) Market Trends
“Enhanced Convenience Through Clinical Decision Support and Interoperability”
- A significant and accelerating trend in the global software as a medical device (SaMD) market is the growing focus on clinical decision support tools and seamless interoperability with electronic health records (EHRs) and connected medical devices. This integration is enabling faster, evidence-based decision-making and improving clinical workflows across care settings
- For instance, leading SaMD platforms are offering real-time diagnostic assistance for conditions such as diabetic retinopathy, cardiovascular risk, and stroke detection. These platforms analyze patient data instantly and support clinicians with actionable insights, reducing diagnostic delays and improving treatment outcomes
- Furthermore, SaMD solutions are increasingly being designed to operate across various digital platforms, including mobile apps, cloud-based systems, and in-hospital terminals—ensuring accessibility and ease of use for both patients and healthcare providers
- The seamless integration of SaMD tools with existing healthcare IT infrastructure allows providers to synchronize patient history, monitor vitals remotely, and streamline data collection. This interoperability is particularly valuable for chronic disease management, where continuous data flow between patient and provider is critical
- This trend towards intelligent, real-time, and connected software-based diagnostics and monitoring systems is fundamentally reshaping the way healthcare is delivered. Companies such as Biofourmis, BrightInsight, and Digital Diagnostics are actively developing SaMD solutions that deliver measurable clinical outcomes and regulatory compliance
- The demand for SaMD solutions that offer remote monitoring, predictive analytics, and real-time alerts is growing rapidly across hospitals, specialty clinics, and home-care environments, as stakeholders seek more efficient, scalable, and patient-centric healthcare solutions
Software as a Medical Device (SaMD) Market Dynamics
Driver
“Growing Need Due to Advancing Digital Health Technologies and Remote Monitoring”
- The increasing demand for remote healthcare solutions, accelerated by the rise in chronic diseases and aging populations, is a significant driver for the growing adoption of Software as a Medical Device (SaMD). These digital solutions enable real-time patient monitoring, diagnostics, and treatment support without requiring traditional hardware integration
- For instance, in April 2024, Roche expanded its digital health portfolio by announcing plans to develop new AI-driven SaMD solutions for personalized cancer therapy decision support, integrating genomic data with clinical records. Such initiatives by key industry players are expected to propel the growth of the global SaMD market during the forecast period
- SaMD platforms offer several advantages, including continuous health monitoring, rapid diagnostics, early disease detection, and better patient engagement—features that are becoming increasingly essential in the context of rising virtual care and home-based medical services
- Furthermore, the global shift toward smart, interoperable health ecosystems—powered by wearable devices, cloud platforms, and mobile health applications—is making SaMD an integral part of digital healthcare infrastructure. These software tools enhance clinical workflows and enable healthcare providers to offer scalable, personalized care
- The convenience of app-based access, data sharing between providers and patients, and compatibility with wearable health devices (such as smartwatches and biosensors) are key factors driving adoption. SaMD solutions are increasingly favored in both hospital and ambulatory care settings due to their ability to improve clinical outcomes and reduce healthcare costs
Restraint/Challenge
“Data Privacy Concerns and Stringent Regulatory Frameworks”
- Despite its benefits, the SaMD market faces significant challenges related to data privacy and regulatory compliance. As these solutions handle sensitive health information and rely heavily on cloud infrastructure, they are vulnerable to cyber threats, unauthorized access, and data breaches
- For instance, reports of privacy breaches involving health apps and wearable-integrated platforms have raised concerns among healthcare providers and patients alike, slowing adoption in some regions. Ensuring secure storage, encryption, and data transmission is critical to safeguarding patient trust
- Companies must navigate complex and evolving regulatory pathways, such as U.S. FDA’s SaMD Pre-Cert Program or EU’s Medical Device Regulation (MDR), which require rigorous clinical validation, real-world evidence, and post-market surveillance for compliance. This can increase product development time and cost
- In addition, the relatively high cost associated with developing and maintaining SaMD products—especially those using advanced technologies such as AI and machine learning—can pose a barrier for smaller digital health startups or healthcare systems in low-resource settings
- Overcoming these challenges will require investment in secure architectures, transparent data practices, and collaboration with regulatory bodies to streamline approval processes. Educating healthcare professionals and patients about data security, alongside developing cost-effective SaMD offerings, will be vital for broader market penetration
Software as a Medical Device (SaMD) Market Scope
The market is segmented on the basis of type and application.
- By Type
On the basis of type, the software as a medical device (SaMD) market is segmented into cloud-based and on-premise solutions. The cloud-based segment dominated the market with the largest revenue share of 65.4% in 2024, driven by the increasing adoption of scalable, interoperable digital healthcare platforms and growing demand for remote patient care. Cloud-based SaMD offers real-time updates, global accessibility, and integration with telemedicine—making it the most preferred deployment model among healthcare providers.
The on-premise segment is projected to grow at a fatstest CAGR of 13.2% from 2025 to 2032, particularly in healthcare organizations that prioritize complete control over data storage and privacy, especially in regions with strict compliance frameworks.
- By Application
On the basis of application, the software as a medical device (SaMD) market is segmented into screening and diagnosis, monitoring and alerting, and chronic disease management. The screening and diagnosis segment held the largest revenue share of 47.8% in 2024, attributed to the rise of AI-enabled diagnostic platforms used for cancer screening, cardiovascular assessments, and imaging analysis. SaMD tools in this area enhance diagnostic precision and speed in clinical settings. The monitoring and alerting segment is expected to witness the fastest CAGR of 25.1% from 2025 to 2032, driven by the increasing adoption of remote patient monitoring systems and wearable-integrated platforms that provide real-time health alerts, particularly for elderly and high-risk patients.
Software as a Medical Device (SaMD) Market Regional Analysis
- North America dominated the software as a medical device (SaMD) market with the largest revenue share of 44.8% in 2024, driven by a growing demand for digital healthcare tools and regulatory support for software-based clinical solutions
- The region's robust health IT infrastructure, high healthcare expenditure, and increasing reliance on remote monitoring and diagnostics tools contribute significantly to this leadership
- The U.S. leads the North American market due to a favorable regulatory environment from the FDA, rapid digital transformation in hospitals, and the presence of key SaMD developers
U.S. Software as a Medical Device (SaMD) Market Insight
The U.S. software as a medical device market accounted for the largest share within North America, contributing 81% of the region’s market revenue in 2024. Growth is driven by the widespread integration of AI in diagnostics, telehealth expansion, and a shift toward value-based care. Companies are focusing on FDA 510(k) clearances and expanding indications for SaMD products in chronic disease management and mental health monitoring.
Europe Software as a Medical Device (SaMD) Market Insight
The Europe software as a medical device market software as a medical device market is projected to grow at a notable CAGR of 21.3% from 2025 to 2032, owing to the implementation of the EU MDR (Medical Device Regulation) and a strong focus on patient data safety and innovation. Countries such as Germany, France, and the U.K. are promoting digital health transformation through reimbursement models and investment in telemedicine platforms.
U.K. Software as a Medical Device (SaMD) Market Insight
The U.K. software as a medical device market is anticipated to grow at a robust CAGR of 20.7% during the forecast period. Key factors include NHS Digital initiatives, growing digital literacy among healthcare professionals, and public trust in AI-powered tools for mental health and chronic disease self-management.
Germany Software as a Medical Device (SaMD) Market Insight
The Germany software as a medical device market is projected to expand at a CAGR of 20.9% from 2025 to 2032. The Digital Healthcare Act (DVG) and the DiGA Fast-Track process are key catalysts, enabling digital health apps to receive reimbursement, thus encouraging innovation and adoption.
Asia-Pacific Software as a Medical Device (SaMD) Market Insight
The Asia-Pacific software as a medical device market is expected to register the fastest CAGR of 21.6% from 2025 to 2032, supported by government digital health campaigns, growing telemedicine usage, and increased smartphone penetration. Countries such as China, Japan, and India are actively investing in AI-driven diagnostics, mHealth apps, and cloud-based SaMD platforms.
Japan Software as a Medical Device (SaMD) Market Insight
The Japan software as a medical device market is growing steadily, influenced by its aging population and strong tech-driven healthcare reforms. The Japanese government’s Society 5.0 initiative supports medical software innovation, and insurers are beginning to reimburse digital therapeutics.
China Software as a Medical Device (SaMD) Market Insight
The China software as a medical device market held the largest market revenue share in Asia-Pacific in 2024 due to strong government support for AI in healthcare, the large patient base, and increased use of smartphone-based health tools. The National Health Commission’s guidelines on AI and digital medical devices have boosted regulatory clarity, supporting rapid domestic SaMD development and adoption.
Software as a Medical Device (SaMD) Market Share
The software as a medical device (SaMD) industry is primarily led by well-established companies, including:
- MindMaze (Switzerland)
- Siemens Healthineers AG (Germany)
- Biofourmis (U.S.)
- Digital Diagnostics Inc. (U.S.)
- Silicon & Software Systems Ltd. (Ireland)
- BrightInsight, Inc. (U.S.)
- Arterys (U.S.)
- Medtronic (Ireland)
- Viz.ai, Inc. (U.S.)
- iSchemaView, Inc. (U.S.)
- Abbott (U.S.)
- Oracle (U.S.)
- 4S Information Systems Ltd. (India)
- Axis Clinical Software, Inc. (U.S.)
- CV Medical Software (U.S.)
Latest Developments in Global Software as a Medical Device (SaMD) Market
- In May 2025, the U.S. Food and Drug Administration (FDA) released updated draft guidance on Artificial Intelligence-Enabled Device Software Functions, which directly impacts the SaMD sector. This guidance introduces a Predetermined Change Control Plan (PCCP), allowing manufacturers to proactively manage algorithm updates post-market. This move signifies regulatory adaptability in line with the dynamic nature of AI-powered SaMDs and aims to accelerate innovation while maintaining safety and efficacy standards
- In February 2025, Biofourmis, a U.S.-based healthtech company, announced the expansion of its FDA-cleared SaMD platform for heart failure remote monitoring to European markets, following CE certification. The company’s AI-driven solution allows real-time patient insights and has demonstrated reduction in hospital readmissions, offering significant value in post-acute and chronic care
- In January 2025, Digital Diagnostics Inc. launched its new SaMD-based autonomous diagnostic platform for diabetic retinopathy screening in primary care settings. Approved by the FDA and now expanding into Asia-Pacific, this tool eliminates the need for specialist intervention at initial diagnosis, addressing accessibility gaps in vision care
- In March 2024, Siemens Healthineers announced a strategic partnership with U.S. hospital networks to implement their AI-powered SaMD imaging platform across radiology departments. This collaboration focuses on real-time decision support for tumor detection and stroke management, enhancing diagnostic accuracy and workflow efficiency
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.