Global Safety Syringes Market By Product (Retractable Safety Syringes and Non Retractable Safety Syringes), Type (Attached Needle and Detachable Needles), Therapy (Insulin and Tuberculosis), End-User (Hospitals, Blood Donation Camps, and Others) - Industry Trends and Forecast to 2030.

Safety Syringes Market Analysis and Size
The increasing focus on needlestick injuries is a major factor driving the growth of the safety syringes market. The growing prevalence of blood-borne diseases, increasing technological advancements, and healthcare expenditures accelerate the safety syringes market growth.
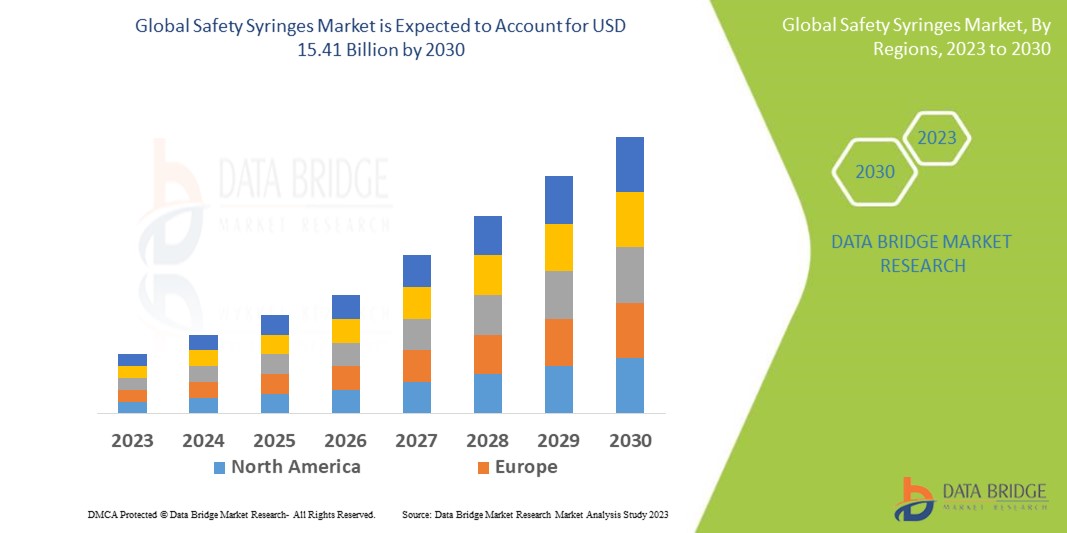
Data Bridge Market Research analyses that the global safety syringes market, which was USD 8.45 billion in 2022, would rocket up to USD 15.41 billion by 2030 and is expected to undergo a CAGR of 7.8 % during the forecast period. The retractable safety syringes segment dominates the product type segment of the safety syringes market owing to the advancements in technology that have led to advancements in syringes for various use. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Safety Syringes Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
By Product (Retractable Safety Syringes and Non Retractable Safety Syringes), Type (Attached Needle and Detachable Needles), Therapy (Insulin and Tuberculosis), End-User (Hospitals, Blood Donation Camps, and Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, Brazil, Argentina, Peru, rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, rest of Asia-Pacific, Saudi Arabia, U.A.E, Egypt, Israel, Kuwait, South Africa, rest of Middle East and Africa
|
|
Market Players Covered
|
BD (U.S), Boston Scientific Corporation (U.S.), B. Braun Melsungen AG (U.S.), Nipro Medical Corporation (Japan), Baxter. (U.S.), Retractable Technologies, Inc. (U.S.), Smiths Medical (U.S.), Medtronic(U.S.), Terumo Corporation, JMI Syringes & Medical Devices Limited (Japan), Revolutions Medical Corporation (U.S.), Verdict Media Limited (U.K.), SOL-Millennium. (U.S.), AxelBio. (U.S.),, Fresenius Kabi AG (Germany), FLEX LTD.(Singapore), Vita Needle Company(U.S.), Novo Nordisk A/S (U.S.) and Henke-Sass Wolf(U.S.)
|
|
Market Opportunities
|
|
Market Definition
It refers to the syringes that are specifically designed to enhance safety and minimize the risk of needlestick injuries and transmission of infectious diseases among healthcare professionals and patients. Safety syringes have built-in features or mechanisms that help prevent accidental needlestick injuries during various medical procedures, such as injections, blood collection, and intravenous drug administration.
These safety features can include retractable needles or sheaths, shielding mechanisms, or needlestick prevention systems that automatically cover or lock the needle after use. Safety syringes protect healthcare workers from accidental needlestick injuries, reduce the risk of exposure to bloodborne pathogens, and promote safe medical practices.
Global Safety Syringes Market Dynamics
Drivers
- Increasing Needlestick Injuries:
Needlestick injuries pose a significant risk to healthcare workers and can lead to the transmission of bloodborne diseases such as HIV, hepatitis B, and hepatitis C. The rising awareness and concern about needlestick injuries have driven the demand for safety syringes designed to prevent accidental needlestick injuries.
- Technological Advancements:
The safety syringe market has witnessed significant technological advancements, leading to the development of innovative and more effective safety mechanisms. Manufacturers have introduced various safety syringes, such as retractable syringes, sheathing syringes, and passive safety syringes, which offer improved protection and ease of use. These technological advancements have contributed to the market growth.
- Growing Focus on Healthcare Worker Safety:
Healthcare organizations and institutions are increasingly focused on protecting the safety and well-being of their employees. Safety syringes are considered essential to healthcare worker safety programs, as they reduce the risk of needlestick injuries. The growing emphasis on healthcare worker safety has boosted the demand for safety syringes.
Opportunities
- Rising Healthcare Expenditure:
Globally, healthcare expenditure has been increasing, driven by population growth, aging populations, and the expansion of healthcare infrastructure. With a higher focus on patient and healthcare worker safety, a portion of this expenditure is allocated to procuring safety syringes. The growing healthcare expenditure provides a favorable market environment for the safety syringes industry.
- Growing Awareness and Education:
Awareness campaigns and educational initiatives regarding the importance of safety syringes and the risks associated with needlestick injuries have driven market growth. Governments, healthcare organizations, and non-profit organizations are actively spreading awareness and educating healthcare workers about the benefits of safe syringes, leading to increased adoption.
Challenges/ Restraints
- Cost considerations:
Safety syringes are generally more expensive than conventional syringes due to the added safety features they incorporate. This cost difference can pose a challenge, particularly in developing countries or regions with limited healthcare budgets. Affordability becomes a significant factor in the widespread adoption of safe syringes.
- Lack of regulatory standards:
Although safety syringes are designed to enhance patient and healthcare worker safety, there is a lack of uniform regulatory standards governing their use and manufacturing. Varying regulations across different countries or regions can create complexities for manufacturers and hinder market growth.
This global safety syringes market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global safety syringes market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In May 2022, Babson Diagnostics, a science-first health care technology company, and BD (Becton, Dickinson, and Company) (NYSE: BDX), a leading global medical technology company, today announced the expansion of a strategic partnership to move blood sample collection into new care settings, including enabling patients to collect blood samples at home for diagnostic testing
- In June 2022, Boston Scientific Corporation announced that it had entered into a definitive agreement with Synergy Innovation Co., Ltd, to purchase its majority stake (approximately 64 percent) of M.I.Tech Co., Ltd, ("M.I.Tech"), a publicly traded Korean manufacturer and distributor of medical devices for endoscopic and urologic procedures
Global Safety Syringes Market Scope
The Global Safety Syringes Market is segmented on the basis product type, type, drugs, therapy, and end users. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
By Product
- Retractable Safety Syringes
- Non Retractable Safety Syringes
By Type
- Attached Needle
- Detachable Needles
By drugs
- Calcineurin Inhibitors
- Melanocyte-Stimulating Hormone
- Other
By Therapy
- Insulin
- Tuberculosis
By end users
- Hospitals
- Blood Donation Camps
- Others
Global Safety Syringes Market Regional Analysis/Insights
The global safety syringes market is analysed and market size insights and trends are provided product type, type, drugs, therapy, and end users as referenced above.
The countries covered in the global safety syringes market report are U.S., Canada, Mexico, Brazil, Argentina, Peru, rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, rest of Asia-Pacific, Saudi Arabia, U.A.E, Egypt, Israel, Kuwait, South Africa, rest of Middle East and Africa.
North America dominates the safety syringes market due to major key players, high disposable income, high healthcare expenditure, and well-developed healthcare sector in this region. Asia-Pacific is expected to grow during the forecast period of 2023-2030 due to the increasing research and development activities, rising investment in the healthcare sector, and growing government support.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The global safety syringes market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for Global Safety Syringes Market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the Global Safety Syringes Market. The data is available for historic period 2010-2020.
Competitive Landscape and Global Safety Syringes Market Share Analysis
The global safety syringes market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Global Safety Syringes Market.
Some of the major players operating in the global safety syringes market are:
- BD (U.S)
- Boston Scientific Corporation (U.S.)
- B. Braun Melsungen AG (U.S.)
- Nipro Medical Corporation (Japan)
- Baxter. (U.S.)
- Retractable Technologies, Inc. (U.S.)
- Smiths Medical (U.S.)
- Medtronic(U.S.)
- Terumo Corporation (Japan)
- JMI Syringes & Medical Devices Limited (U.K.)
- Revolutions Medical Corporation (U.S.)
- Verdict Media Limited (U.K.)
- SOL-Millennium. (U.S.)
- AxelBio. (U.S.)
- Fresenius Kabi AG (Germany)
- FLEX LTD.(Singapore)
- Vita Needle Company(U.S.)
- Novo Nordisk A/S (U.S.)
- Henke-Sass Wolf(U.S.)
SKU-

