Global Rapid Diagnostic Tests (RDT) Market, By Product Type (Consumables and Kits, Instruments and Others), Mode (Professional Rapid Diagnostic Test Product and Over-The-Counter [OTC] Rapid Diagnostic Test Product), Technology (PCR-Based, Flow-Through Assays, Lateral Flow Immunochromatographic Assays, Agglutination Assay, Microfluidics, Substrate Technology and Others), Modality (Laboratory Based Test and Non-Laboratory Based Test), Age Group (Adult and Pediatric), Test Type (Determining Confirmation, Serological Testing and Viral Sequencing), Approach (In-Vitro Diagnostic, Molecular Diagnostic), Specimen (Swab, Blood, Urine, Saliva, Sputum and Others), Application (Infectious Disease Testing, Glucose Monitoring, Cardiology Testing, Oncology Testing, Cardiometabolic Testing, Drugs-of-Abuse Testing, Pregnancy & Fertility Testing, Toxicology Testing, Others), End User (Hospital & Clinic, Diagnostic Laboratory, Home Care Setting, Research and Academic Institutes and Others), Distribution Channel (Direct Tender, Retail Sales, Others), Country (U.S., Canada, and Mexico, Germany, France, U.K., Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, and the Rest of Europe, China, Japan, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines and Rest of Asia-Pacific, South Africa, Nigeria, Ethiopia and Rest of Africa) – Industry Trends and Forecast to 2029
Market Analysis and Insights: Global Rapid Diagnostic Tests (RDT) Market

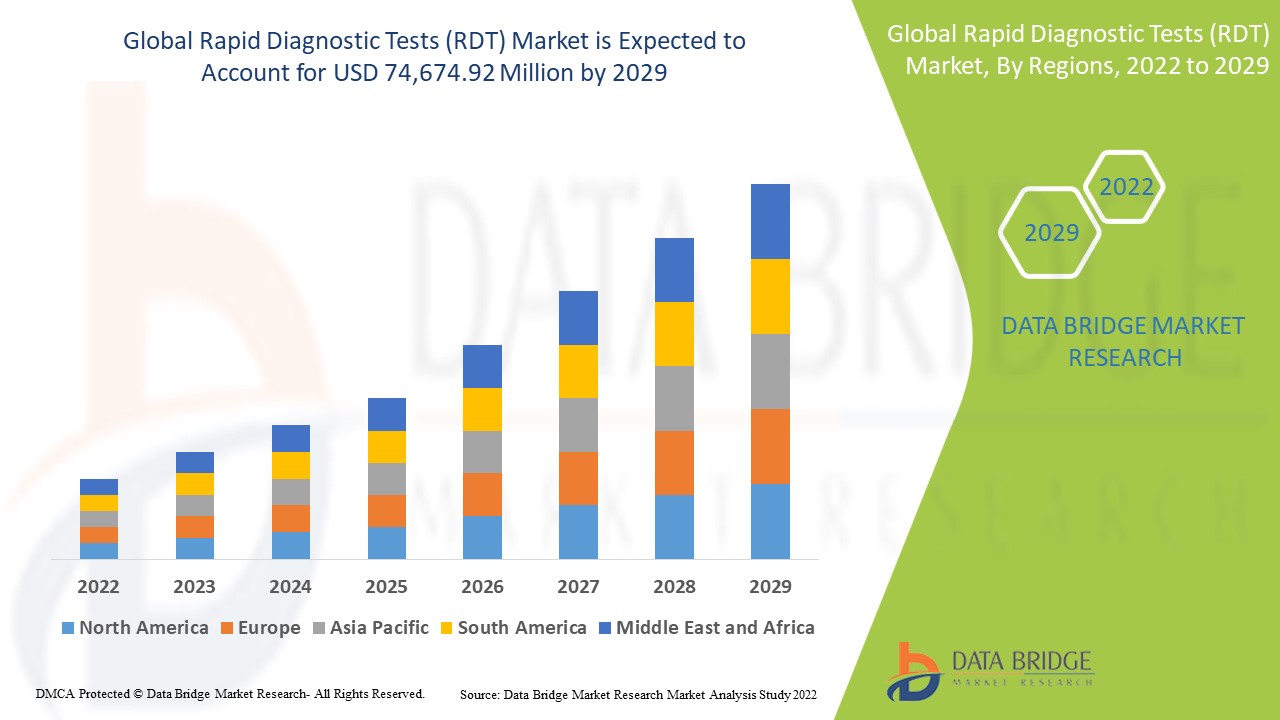
The global rapid diagnostic tests (RDT) market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 9.3% in the forecast period of 2022 to 2029 and is expected to reach USD 74,674.92 million by 2029 from USD 38,363.51 million in 2021. The rise in incidence in chronic diseases and technological advancements in rapid diagnostic tests are likely to be the major drivers which propel the demand of the market in the forecast period.
The rapid diagnostic tests, also called rapid tests, are easy-to-use tests that offer quick results, usually in less than 20 minutes. Unlike other standard and conventional tests, where the diagnosis and sampling are sent to the laboratory, the results obtained from rapid diagnostic test kits are provided at the point of care. The point of care is a place where the patients get cured. The point of care can be a provider's office, a clinic, or a patient's own house.
The RDTs are applicable for diagnosing and testing infectious diseases such as COVID-19, cardiology diseases, oncology, pregnancy, fertility testing, toxicology testing, drugs-of-abuse testing, cardiometabolic testing, and glucose monitoring.
The factors expected to drive the global rapid diagnostic tests (RDT) market are the increase in incidence in chronic diseases, rise in geriatric population, technological developments in the rapid diagnostic tests. However, the factors which are expected to restrain the market are the rise in the cost of rapid diagnostics, product recalls faced in rapid diagnostic tests, and lack of awareness about the use of rapid diagnostics. Moreover, the strategic initiatives by market players and the rise in healthcare expenditure are expected to create opportunities for the global rapid diagnostic tests (RDT) market growth. However, the need for skilled labor for sample collection and the late approval associated with product launches may significantly challenge market growth.
The global rapid diagnostic tests (RDT) market report provides details of market share, new developments, and product pipeline analysis, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief; our team will help you create a revenue impact solution to achieve your desired goal.
Global Rapid Diagnostic Tests (RDT) Market Scope and Market Size

The global rapid diagnostic tests (RDT) market is categorized into eleven notable segments based on product type, mode, technology, modality, age group, test type, approach, specimen, application, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of product type, the global rapid diagnostic tests (RDT) market is segmented into consumables and kits, instruments, and others. In 2022, the consumables and kits segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the ease of use and availability of test kits.
- On the basis of mode, the global rapid diagnostic tests (RDT) market is segmented into professional rapid diagnostic test product, and over-the-counter [OTC] rapid diagnostic test product. In 2022, the professional rapid diagnostic test product segment is expected to dominate the global rapid diagnostic tests (RDT) market due to accuracy and increased use in points of care, such as homes.
- On the basis of technology, the global rapid diagnostic tests (RDT) market is segmented into PCR-based, flow-through assays, lateral flow immunochromatographic assays, agglutination assay, microfluidics, substrate technology, and others. In 2022, the PCR-based segment is expected to dominate the global rapid diagnostic tests (RDT) market due to speed, reliability, delivery of results at a faster rate, and quantitative capacity.
- On the basis of modality, the global rapid diagnostic tests (RDT) market is segmented into laboratory based test and non-laboratory based test. In 2022, the laboratory based test segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the safety, availability of laboratories, following the regulatory guidelines, and cost savings.
- On the basis of age group, the global rapid diagnostic tests (RDT) market is segmented into adult and pediatric. In 2022, the adult segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the increase in the geriatric population and chronic infectious diseases.
- On the basis of test type, the global rapid diagnostic tests (RDT) market is segmented into determining confirmation, serological testing, and viral sequencing. In 2022, the determining confirmation segment is expected to dominate the global rapid diagnostic tests (RDT) market since it is necessary to determine the cause of infectious diseases since the diagnosis by the RDTs provides confirmed results of the diagnosis of infectious disease.
- On the basis of approach, the global rapid diagnostic tests (RDT) market is segmented into in-vitro diagnostic and molecular diagnostic. In 2022, the in-vitro diagnostic segment is expected to dominate the global rapid diagnostic tests (RDT) market due to its ability to detect infectious diseases, such as the COVID-19, detection of pregnancy and fertility disorders, and its applicability in precision medicine.
- On the basis of specimen, the global rapid diagnostic tests (RDT) market is segmented into swab, blood, urine, saliva, sputum, and others. In 2022, the swab segment is expected to dominate the global rapid diagnostic tests (RDT) market, due to its increased preference by the patients and advancements in serology, for the collection of swabs.
- On the basis of application, the global rapid diagnostic tests (RDT) market is segmented into infectious disease testing, glucose monitoring, cardiology testing, oncology testing, cardiometabolic testing, drugs-of-abuse testing, pregnancy & fertility testing, toxicology testing, and others. In 2022, the infectious disease testing segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the rise in cases of chronic infectious disease and government funding for the use of rapid diagnostics.
- On the basis of end user, the global rapid diagnostic tests (RDT) market is segmented into hospital & clinic, diagnostic laboratory, home care setting, research and academic institutes, and others. In 2022, the hospital and clinic segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the availability of advanced Rapid Diagnostic Test (RDT)kits in hospitals and the rise in disposable income.
- On the basis of distribution channel, the global rapid diagnostic tests (RDT) market is segmented into direct tender, retail sales, and others. In 2022, the direct tender segment is expected to dominate the global rapid diagnostic tests (RDT) market due to the rise in contractual agreements between manufacturers and distributors for rapid diagnostic tests and equity in the tendering process.
Global Rapid Diagnostic Tests (RDT) Market Country Level Analysis
The global rapid diagnostic tests (RDT) market is analyzed, and market size information is provided by country, product type, mode, technology, modality, age group, test type, approach, specimen, application, end user, and distribution channel.
The countries covered in the global rapid diagnostic tests (RDT) market report are the U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Russia, Poland, Turkey, Switzerland, Netherlands, Hungary, Austria, Norway, Ireland, Lithuania, rest of Europe, China, Japan, India, Australia, South Korea, Singapore, Thailand, Malaysia, Indonesia, Philippines, Vietnam, rest of Asia-Pacific, South Africa, Nigeria, Ethiopia, Rest of Africa.
The U.S. is expected to dominate the global rapid diagnostic tests (RDT) market due to the presence of FDA-approved rapid diagnostic tests (RDT), presence of health remuneration policies, rise in infectious diseases, and rise in the elderly population. The consumables and kits segment is projected to dominate the U.S., since it's cost-effective. Germany is expected to dominate the global rapid diagnostic tests (RDT) in the Europe region due to untapped opportunities in Germany for rapid diagnostic tests (RDT) and the increased prevalence of cases of diabetic foot ulcers. Additionally, China is expected to dominate the global rapid diagnostic tests (RDT) in the Asia-Pacific region due to increased awareness regarding the treatment for infectious disease and favorable reimbursement scenarios in China
The country section of the report also provides individual market impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of sales channels are considered while providing forecast analysis of the country data.
Growth of Rapid Diagnostic Tests (RDT) in Emerging Economies and Strategic Initiatives by Market Players Create New Opportunities in the Global Rapid Diagnostic Tests (RDT) Market
The global rapid diagnostic tests (RDT) market also provides you with detailed market analysis for every country growth in a particular industry with wound debridement device sales, the impact of advancement in the rapid diagnostic tests (RDT), and changes in regulatory scenarios with their support for the rapid diagnostic tests (RDT) market. The data is available for the historical period 2011 to 2020.
Competitive Landscape and Global Rapid Diagnostic Tests (RDT) Market Share Analysis
The global rapid diagnostic tests (RDT) market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width, and breadth, application dominance, technology lifeline curve. The above data points are only related to the company's focus on the global rapid diagnostic tests (RDT) market.
Some of the major companies in the global rapid diagnostic tests (RDT) market are Abbott, Danaher, Cellex, AdvaCare Pharma, Access Bio, Cardinal Health, Bio-Rad Laboratories, Inc., BD, F. Hoffmann-La Roche Ltd, bioMérieux SA, InBios International, Inc., Gnomegen LLC, QIAGEN, Quidel Corporation, Chembio Diagnostics Systems, Inc., Siemens Healthcare Gmbh (A Subsidiary Siemens Healthineers AG), PerkinElmer Inc., Sekisui Diagnostics, Fujirebio (A Subsidiary of H.U. Group), PTS Diagnostics, LamdaGen Corporation, Werfen, Nova Biomedical, Trinity Biotech, Sysmex Europe GmbH (A Subsidiary of Sysmex Corporation), Luminex Corporation. A DiaSorin Company, MEGAKOR DIAGNOSTK GMBH, among others. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The strategic initiatives by market players and new technological advancements for rapid diagnostic tests (RDT) are bridging the gap for diagnosis and treatment.
For instance,
- In June 2021, Bio-Rad Laboratories, Inc. partnered with Seegene, Inc., a global leader in multiplex molecular diagnostics for the clinical development and commercialization of infectious disease molecular diagnostic products. This partnership has allowed the company to increase its sales and product line globally
- In March 2020, Danaher Completed the acquisition of the Biopharma business from General Electric. In the future, this business will be called Cytiva and known as a standalone operating company within Danaher's Life Sciences segment. Thus, these acquisition helps the company diversify their segment portfolio
Collaboration, joint ventures, and other strategies by the market players enhance the company in the Asia-Pacific rapid diagnostic tests (RDT) market, which also benefits the organization to improve their offering for the market.
SKU-

