Global Pregabalin Market
Market Size in USD Million
CAGR :
% 
 USD
853.86 Million
USD
1,126.98 Million
2024
2032
USD
853.86 Million
USD
1,126.98 Million
2024
2032
| 2025 –2032 | |
| USD 853.86 Million | |
| USD 1,126.98 Million | |
|
|
|
|
Pregabalin Market Size
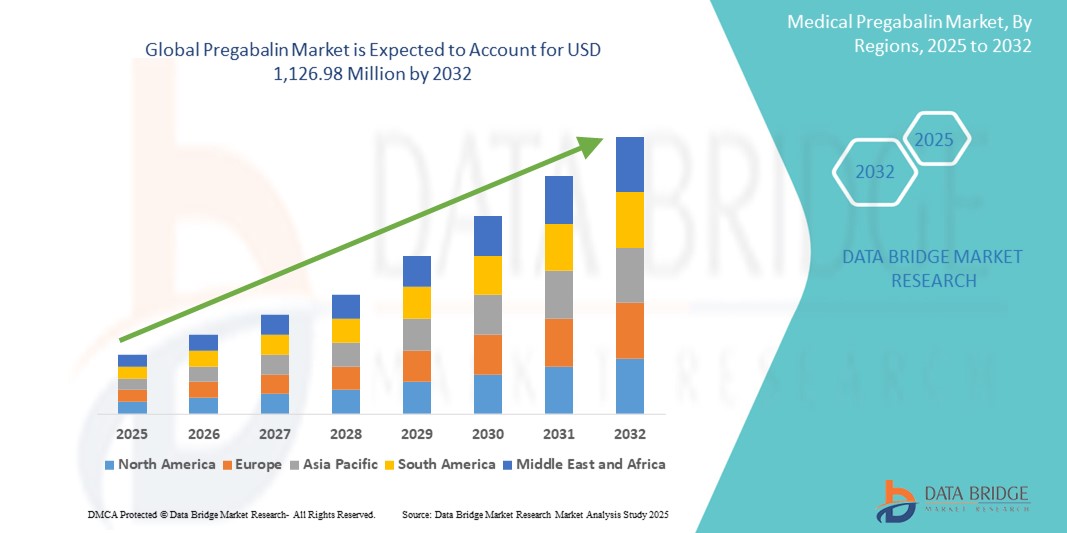
- The global pregabalin market size was valued at USD 853.86 Million in 2024 and is expected to reach USD 1,126.98 Million by 2032, at a CAGR of 3.53% during the forecast period
- The market growth is largely fueled by the rising incidence of neuropathic pain, epilepsy, and generalized anxiety disorder (GAD), coupled with growing awareness and diagnosis rates across both developed and developing regions. This increasing patient pool is directly contributing to the heightened demand for pregabalin as a first-line treatment option
- Furthermore, rising consumer demand for effective, fast-acting, and well-tolerated therapeutic options is establishing Pregabalin as the preferred choice across multiple neurological and pain-related conditions. These converging factors are accelerating the uptake of Pregabalin solutions, thereby significantly boosting the industry's growth
Pregabalin Market Analysis
- Pregabalin, an anticonvulsant and anxiolytic medication, plays a critical role in the treatment of neuropathic pain, epilepsy, fibromyalgia, and generalized anxiety disorder (GAD). It is increasingly vital in modern neurology and pain management protocols due to its fast-acting relief, minimal drug interaction profile, and effectiveness across multiple indications
- The escalating demand for pregabalin is primarily fueled by the growing global burden of chronic pain and neurological disorders, along with increased diagnosis rates and healthcare accessibility in emerging economies. Rising geriatric populations, who are more prone to such conditions, further contribute to market expansion
- North America dominated the pregabalin market with the largest revenue share of 42.8% in 2024, driven by strong prescription volumes in the U.S., a mature healthcare system, and robust insurance coverage. The region benefits from high awareness levels, advanced diagnostic capabilities, and established market players focusing on neuropathic and psychiatric disorders
- Asia-Pacific is expected to be the fastest-growing region in the pregabalin market, projected to expand at a CAGR of 8.9% from 2025 to 2032, fueled by rapid urbanization, rising healthcare spending, and increasing patient awareness. Countries such as China and India are witnessing a surge in epilepsy and diabetic neuropathy cases, supporting wider adoption of Pregabalin
- The neuropathic pain segment dominated the pregabalin market with a revenue share of 47.3% in 2024, owing to the high prevalence of diabetes-induced nerve damage, post-herpetic neuralgia, and chemotherapy-induced neuropathy. Pregabalin’s FDA-approved use for multiple neuropathic conditions ensures continued demand across various patient groups
Report Scope and Pregabalin Market Segmentation
|
Attributes |
Pregabalin Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pregabalin Market Trends
“Growing Importance of AI in Drug Discovery and Personalized Therapeutics””
- A significant and accelerating trend in the global pregabalin market is the integration of artificial intelligence (AI) in drug discovery, clinical trial optimization, and personalized medicine. This technological advancement is improving the efficiency and precision of developing pregabalin-based therapies, particularly in the treatment of neuropathic pain, epilepsy, and anxiety disorders
- For instance, leading pharmaceutical companies are using AI platforms to analyze patient data, predict treatment responses, and optimize dosage regimens for pregabalin. Such developments are enabling more targeted and effective treatment plans, improving outcomes and reducing side effects
- AI integration is also helping identify novel indications for pregabalin through data mining and pattern recognition in real-world evidence. This not only expands the therapeutic potential of the drug but also enhances post-market surveillance and pharmacovigilance
- The use of predictive analytics powered by AI is streamlining clinical trial design and recruitment for new pregabalin formulations, such as extended-release versions or combination therapies, significantly reducing time-to-market
- This trend towards more intelligent, data-driven drug development is fundamentally transforming the pharmaceutical landscape. As a result, companies such as Pfizer and Teva Pharmaceuticals are investing heavily in AI partnerships to accelerate innovation in the pregabalin segment
Pregabalin Market Dynamics
Driver
“Growing Need Due to Rising Neuropathic Disorders and Personalized Pain Management”
- The increasing prevalence of neuropathic pain disorders, epilepsy, and generalized anxiety, coupled with a shift toward more personalized medicine, is a significant driver for the heightened demand for Pregabalin
- For instance, in April 2024, Pfizer Inc., one of the primary manufacturers of Pregabalin, announced a strategic expansion into emerging markets across Asia and Latin America, aiming to improve access to neuropathic pain medications. Such initiatives by key players are expected to accelerate the growth of the Pregabalin industry during the forecast period
- As healthcare providers and patients become more aware of the long-term consequences of unmanaged nerve pain and epilepsy, Pregabalin’s proven efficacy in providing symptomatic relief makes it a first-line treatment choice in multiple therapeutic guidelines
- Furthermore, the growing acceptance of centralized prescription models, increased mental health awareness, and chronic pain management strategies are making Pregabalin an integral part of multi-modal treatment protocols in hospitals and specialty clinics
- The convenience of once or twice daily dosing, availability in multiple dosage forms (capsules, oral solution), and generally favorable side-effect profile contribute to Pregabalin’s strong adoption in both developed and developing regions. The rising geriatric population and the associated increase in neuropathic conditions further amplify this demand
Restraint/Challenge
“Patent Expiry, Regulatory Pressure, and Risk of Abuse Potential”
- The expiration of key patents for branded pregabalin, such as Lyrica, has led to increased generic competition, putting downward pressure on pricing and margins for original manufacturers
- For instance, since 2019, multiple generic players have launched lower-cost alternatives in Europe and the U.S., which—while increasing access—have also introduced concerns over market saturation and profitability for premium brands
- Another significant challenge is the growing regulatory scrutiny related to the misuse and abuse potential of Pregabalin, especially in combination with opioids or other CNS depressants. Several countries, including the U.K. and some EU members, have moved to reclassify Pregabalin as a controlled substance due to rising misuse cases
- To address these challenges, pharmaceutical companies must focus on physician education, proper labeling, and controlled marketing strategies. In addition, the need for more rigorous post-marketing surveillance is essential to mitigate abuse risks
- Although cost-effective generics support market volume growth, the reduced profitability and stricter regulations create headwinds that need strategic navigation to ensure long-term sustainability in the Pregabalin market
Pregabalin Market Scope
The pregabalin market is segmented into five notable segments based on dosage form, application, drug class, end-users, and distribution channel.
• By Dosage Form
On the basis of dosage form, the pregabalin market is segmented into oral capsule, oral solution, oral tablet, and extended release. The oral capsule segment dominated the largest market revenue share of 46.8% in 2024, owing to its wide prescription base, ease of administration, and high patient adherence. Capsules are also favored for their stability and extended shelf life.
The extended-release segment is anticipated to witness the fastest growth rate of 7.9% from 2025 to 2032, driven by increasing demand for once-daily formulations that enhance patient compliance, particularly among individuals managing chronic neuropathic pain and generalized anxiety disorder.
• By Application
On the basis of application, the pregabalin market is segmented into epilepsy, neuropathic pain, anxiety disorder, and others. The neuropathic pain segment accounted for the largest market revenue share of 47.3% in 2024, driven by the growing global burden of diabetes, cancer, and spinal cord injuries—all of which contribute to neuropathic pain. Pregabalin's strong clinical efficacy in pain modulation makes it the preferred choice.
The anxiety disorder segment is expected to witness the fastest CAGR of 8.4% from 2025 to 2032, attributed to rising awareness around mental health and an increasing number of prescriptions in off-label usage for generalized anxiety treatment, especially in Europe and Asia-Pacific.
• By Drug Class
On the basis of drug class, the pregabalin market is segmented into fibromyalgia agents, anticonvulsants, and others. The anticonvulsants segment dominated the market with a revenue share of 49.6% in 2024, as Pregabalin is widely prescribed as a second-generation antiepileptic drug.
The fibromyalgia agents segment is projected to register the fastest CAGR of 7.2% during the forecast period, fueled by growing diagnoses and awareness about fibromyalgia, especially in North America and Western Europe, where pregabalin remains one of the few FDA-approved medications for the condition.
• By End-User
On the basis of end-users, the pregabalin market is segmented into hospitals, specialty clinics, homecare, and others. The hospitals segment held the largest market share of 41.3% in 2024, driven by the high volume of inpatient prescriptions and widespread usage in acute care settings.
The homecare segment is expected to grow at the fastest CAGR of 9.1% between 2025 and 2032, supported by an aging population, rising preference for home-based treatments, and increased accessibility of remote medical guidance.
• By Distribution Channel
On the basis of distribution channel, the pregabalin market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The retail pharmacy segment captured the largest revenue share of 47.9% in 2024, reflecting strong patient access and convenience in obtaining repeat prescriptions for chronic conditions.
The online pharmacy segment is forecasted to experience the fastest CAGR of 10.6% from 2025 to 2032, owing to the rapid digitization of healthcare, improved e-commerce infrastructure, and growing patient preference for discreet, home-delivered medication services.
Pregabalin Market Regional Analysis
- North America dominated the pregabalin market with the largest revenue share of 42.8% in 2024, driven by high prevalence of neuropathic pain and anxiety disorders, growing awareness about fibromyalgia, and widespread access to healthcare services and prescription drugs
- The region benefits from favorable reimbursement policies, a well-established pharmaceutical supply chain, and robust clinical research infrastructure that support the expansion of Pregabalin prescriptions across multiple indications
- In addition, the increasing geriatric population, coupled with a high diagnosis rate for chronic neurological conditions, continues to propel market demand
U.S. Pregabalin Market Insight
The U.S. pregabalin market captured the largest revenue share of 85% in 2024 within North America, attributed to the country’s strong healthcare infrastructure, high rate of chronic pain diagnosis, and a rising number of off-label Pregabalin prescriptions for anxiety disorders and sleep disturbances. The availability of both branded and generic versions enhances accessibility, while a growing trend toward outpatient and homecare services sustains continued market demand.
Europe Pregabalin Market Insight
The Europe pregabalin market is projected to expand at a substantial CAGR throughout the forecast period, fueled by increasing prevalence of epilepsy and neuropathic pain, and growing awareness about mental health disorders. Countries such as Germany, the U.K., and France are leading in terms of prescription volume due to supportive regulatory frameworks and the presence of major generic manufacturers. Additionally, the demand for cost-effective generics is supporting consistent market growth.
U.K. Pregabalin Market Insight
The U.K. pregabalin market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a high incidence of fibromyalgia and diabetic neuropathy. The country’s National Health Service (NHS) guidelines encourage the use of generic pregabalin, ensuring wide patient coverage. Additionally, mental health initiatives and rising general practitioner (GP) consultations for anxiety and sleep disorders are contributing to increased Pregabalin prescriptions.
Germany Pregabalin Market Insight
The Germany pregabalin market is expected to expand at a considerable CAGR, supported by strong insurance coverage for chronic conditions and a growing population of elderly patients susceptible to epilepsy and neuralgia. Germany’s focus on clinical efficacy and strict pharmaceutical regulations favor proven therapies such as Pregabalin. The shift toward outpatient neurological care also provides opportunities for increased Pregabalin use.
Asia-Pacific Pregabalin Market Insight
The Asia-Pacific pregabalin market is poised to grow at the fastest CAGR of 8.9% during 2025–2032, driven by rapidly increasing diabetic population, improved access to healthcare, and rising awareness of neuropathic disorders. In countries such as China, India, and Japan, generic drug production has scaled rapidly, making pregabalin more affordable and widely available. Additionally, increasing mental health diagnoses and growing insurance penetration are further fueling market growth.
Japan Pregabalin Market Insight
The Japan pregabalin market is gaining momentum due to a significant elderly population, high technological advancement in healthcare delivery, and increasing prevalence of chronic pain and epilepsy. Physicians in Japan prefer pregabalin for its effectiveness in treating peripheral neuropathy and post-herpetic neuralgia. Moreover, the government’s support for mental health awareness is contributing to a rise in prescriptions related to generalized anxiety disorder.
China Pregabalin Market Insight
The China pregabalin market accounted for the largest revenue share in Asia-Pacific in 2024, due to the country’s expanding middle class, growing prevalence of diabetes and cancer, and rapid healthcare infrastructure development. Local pharmaceutical companies have increased the production of affordable generic pregabalin, expanding its accessibility across both urban and rural regions.In addition, strong government focus on chronic disease management and pharmaceutical innovation further boosts market potential.
Pregabalin Market Share
The pregabalin industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Zydus Cadila (India)
- Lupin (India)
- Amneal Pharmaceuticals LLC. (U.S.)
- Cipla Inc. (U.S.)
- Torrent Pharmaceuticals Ltd. (India)
- Aurobindo Pharma Limited (India)
- Glenmark Pharmaceuticals Limited (India)
- Medley Pharmaceuticals Ltd. (India)
- Genesisbiotec (India)
- Biomax Biotechnics (India)
- Olon S.p.A. (Italy)
- HIKAL Ltd. (India)
Latest Developments in Global Pregabalin Market
- In February 2024, Pfizer Inc., the original developer of Lyrica (brand name for Pregabalin), announced expanded availability of its generic Pregabalin in several emerging markets including Latin America and Southeast Asia. This move is aimed at improving access to essential neuropathic pain and epilepsy treatments in lower-income countries, thereby reinforcing Pfizer’s leadership in the global CNS therapeutics segment
- In January 2024, Teva Pharmaceutical Industries Ltd. launched a new extended-release formulation of Pregabalin in the U.S. market. Designed for once-daily dosing, this formulation aims to improve patient adherence in managing chronic neuropathic pain and fibromyalgia, particularly among elderly patients
- In October 2023, Dr. Reddy’s Laboratories received FDA approval for its Pregabalin extended-release tablets, 165 mg and 330 mg, marking a significant step in the company’s strategy to diversify its complex generics portfolio in the U.S. market
- In August 2023, Sun Pharmaceutical Industries Ltd. initiated clinical trials in India for a combination therapy of Pregabalin and Duloxetine, targeting treatment-resistant neuropathic pain. The trial aims to evaluate the synergistic effect of dual therapy on diabetic neuropathy and fibromyalgia
- In May 2023, Zydus Lifesciences introduced a novel fixed-dose combination (FDC) of Pregabalin and Nortriptyline in India. This FDC is designed to offer comprehensive pain relief in patients suffering from peripheral neuropathic conditions, enhancing Zydus' neurology product portfolio
- In March 2023, Lupin Limited launched Pregabalin oral solution in the European market following the EMA's approval. The new formulation is intended for patients with swallowing difficulties, particularly in the geriatric and pediatric population
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL PREGABALIN MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL PREGABALIN MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL PREGABALIN MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL PREGABALIN MARKET, BY DRUG TYPE
15.1 OVERVIEW
15.2 GENERICS
15.3 BRANDED
15.3.1 LYRICA
15.3.2 LYRICA CR
15.3.3 OTHERS
15.3.3.1. MARKET VALUE (USD MN)
15.3.3.2. MARKET VOLUME (SU)
15.3.3.3. AVERAGE SELLING PRICE (USD)
16 GLOBAL PREGABALIN MARKET, BY DRUG CLASS
16.1 OVERVIEW
16.2 EXTENDED RELEASE
16.3 REGULAR RELEASE
17 GLOBAL PREGABALIN MARKET, BY DOSAGE FORM
17.1 OVERVIEW
17.2 ORAL CAPSULE
17.2.1 100 MG
17.2.2 150 MG
17.2.3 200 MG
17.2.4 225 MG
17.2.5 25 MG
17.2.6 300 MG
17.2.7 50 MG
17.2.8 75 MG
17.2.9 OTHERS
17.3 ORAL TABLET
17.3.1 165 MG
17.3.2 330 MG
17.3.3 82.5 MG
17.3.4 OTHERS
17.4 ORAL SOLUTION
17.5 OTHERS
18 GLOBAL PREGABALIN MARKET, BY INDICATION
18.1 OVERVIEW
18.2 NEUROPATHY
18.2.1 DIABETIC PERIPHERAL NEUROPATHY
18.2.2 PERIPHERAL NEUROPATHY
18.2.3 SMALL FIBER NEUROPATHY
18.2.4 PERIPHERAL NEUROPATHIC PAIN
18.2.5 CENTRAL NEUROPATHIC PAIN
18.2.6 OTHERS
18.3 EPILEPSIES
18.4 FIBROMYALGIA
18.5 POST HERPETIC NEURALGIA
18.6 GENERALISED ANXIETY DISORDER
18.7 COUGH & CHRONIC REFRACTORY CONDITIONS
18.8 CHRONIC PRUITUS
18.9 OTHERS
19 GLOBAL PREGABALIN MARKET, BY AGE GROUP
19.1 OVERVIEW
19.2 ADULT
19.3 GERIATRIC
20 GLOBAL PREGABALIN MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.2.1 PUBLIC
20.2.2 PRIVATE
20.3 SPECIALTY CLINICS
20.4 ACADEMIC & RESEARCH INSTITUTIONS
20.5 HOME HEALTHCARE
20.6 OTHERS
21 GLOBAL PREGABALIN MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDER
21.3 RETAIL SALES
21.3.1 HOSPITAL PHARMACY
21.3.2 ONLINE PHARMACY
21.3.3 MEDICINE STORES
21.4 OTHERS
22 GLOBAL PREGABALIN MARKET, BY GEOGRAPHY
GLOBAL PREGABALIN MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.1 NORTH AMERICA
22.1.1 U.S.
22.1.2 CANADA
22.1.3 MEXICO
22.2 EUROPE
22.2.1 GERMANY
22.2.2 FRANCE
22.2.3 U.K.
22.2.4 HUNGARY
22.2.5 LITHUANIA
22.2.6 AUSTRIA
22.2.7 IRELAND
22.2.8 NORWAY
22.2.9 POLAND
22.2.10 ITALY
22.2.11 SPAIN
22.2.12 RUSSIA
22.2.13 TURKEY
22.2.14 NETHERLANDS
22.2.15 SWITZERLAND
22.2.16 REST OF EUROPE
22.3 ASIA-PACIFIC
22.3.1 JAPAN
22.3.2 CHINA
22.3.3 SOUTH KOREA
22.3.4 INDIA
22.3.5 AUSTRALIA
22.3.6 SINGAPORE
22.3.7 THAILAND
22.3.8 MALAYSIA
22.3.9 INDONESIA
22.3.10 PHILIPPINES
22.3.11 VIETNAM
22.3.12 REST OF ASIA-PACIFIC
22.4 SOUTH AMERICA
22.4.1 BRAZIL
22.4.2 ARGENTINA
22.4.3 PERU
22.4.4 REST OF SOUTH AMERICA
22.5 MIDDLE EAST AND AFRICA
22.5.1 SOUTH AFRICA
22.5.2 GLOBAL
22.5.3 UAE
22.5.4 EGYPT
22.5.5 KUWAIT
22.5.6 ISRAEL
22.5.7 REST OF MIDDLE EAST AND AFRICA
22.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL PREGABALIN MARKET, SWOT AND DBMR ANALYSIS
24 GLOBAL PREGABALIN MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
24.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
24.6 MERGERS & ACQUISITIONS
24.7 NEW PRODUCT DEVELOPMENT & APPROVALS
24.8 EXPANSIONS
24.9 REGULATORY CHANGES
24.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL PREGABALIN MARKET, COMPANY PROFILE
25.1 PFIZER INC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 VIATRIS INC.
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 ADVACARE PHARMA
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 ALEMBIC PHARMACEUTICALS
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 ALKEM LABORATORIES LTD
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 LUPIN
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 AMNEAL PHARMACEUTIALS LLC.
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 APOTEX INC.
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 CELLTRIONPHARMA INC.
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 DR REDDY LABORATORIES LTD.
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 ESKAYEF PHARMACEUTICALS LIMITED
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 CAMBER PHARMACEUTICALS INC.
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 VIVANTA GENERICS (MSN GROUP)
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 CIPLA
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 MARKSANS PHARMALTD.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 SCIEGEN PHARMACEUTICALS, INC.
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 LAURUS LABS LIMITED
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 STRIDES PHARMA SCIENCE LIMITED
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 SUN PHARMACEUTICALS INDUSTRIES LTD.
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 TEVA PHARMACEUTICALS USA, INC.
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 ADALVO LIMITED
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
25.22 SHANGHAI PHARMA HOLDINGS CO., LTD.
25.22.1 COMPANY OVERVIEW
25.22.2 REVENUE ANALYSIS
25.22.3 GEOGRAPHIC PRESENCE
25.22.4 PRODUCT PORTFOLIO
25.22.5 RECENT DEVELOPMENTS
25.23 AUROBINDO PHARMA USA
25.23.1 COMPANY OVERVIEW
25.23.2 REVENUE ANALYSIS
25.23.3 GEOGRAPHIC PRESENCE
25.23.4 PRODUCT PORTFOLIO
25.23.5 RECENT DEVELOPMENTS
25.24 HETERO HEALTHCARE LIMITED
25.24.1 COMPANY OVERVIEW
25.24.2 REVENUE ANALYSIS
25.24.3 GEOGRAPHIC PRESENCE
25.24.4 PRODUCT PORTFOLIO
25.24.5 RECENT DEVELOPMENTS
25.25 ACCORD HEALTHCARE B.V.( INTAS PHARMACEUTICALS)
25.25.1 COMPANY OVERVIEW
25.25.2 REVENUE ANALYSIS
25.25.3 GEOGRAPHIC PRESENCE
25.25.4 PRODUCT PORTFOLIO
25.25.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.