Global Pleuropulmonary Blastoma Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
680.61 Million
USD
990.34 Million
2025
2033
USD
680.61 Million
USD
990.34 Million
2025
2033
| 2026 –2033 | |
| USD 680.61 Million | |
| USD 990.34 Million | |
|
|
|
|
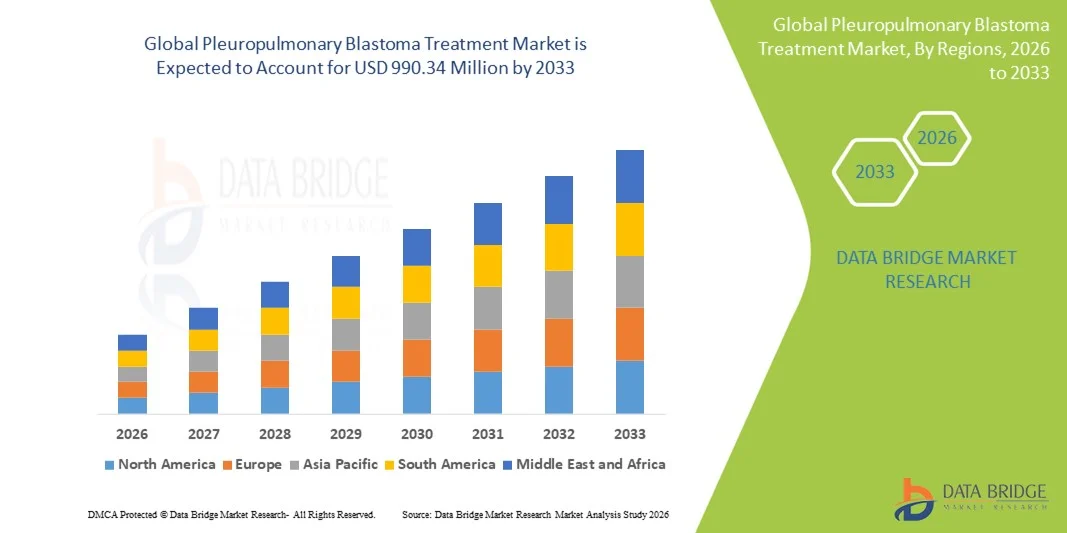
Pleuropulmonary Blastoma Treatment Market Size
- The global pleuropulmonary blastoma treatment market size was valued at USD 680.61 million in 2025 and is expected to reach USD 990.34 million by 2033, at a CAGR of 4.80% during the forecast period
- The market growth is largely driven by increasing awareness of rare pediatric cancers, improving diagnostic capabilities, and the growing adoption of multimodal treatment approaches, including surgery, chemotherapy, and supportive therapies across specialized healthcare settings
- Furthermore, rising investments in rare disease research, expanding access to advanced pediatric oncology care, and ongoing clinical efforts to improve survival outcomes are positioning pleuropulmonary blastoma treatments as a critical focus area, thereby significantly supporting the market’s overall growth trajectory
Pleuropulmonary Blastoma Treatment Market Analysis
- Pleuropulmonary blastoma treatments, encompassing surgical intervention, chemotherapy, and supportive care, are critical components of pediatric oncology due to the aggressive and rare nature of the disease, with treatment strategies increasingly guided by early diagnosis and risk-based clinical protocols
- The growing demand for pleuropulmonary blastoma treatment is primarily driven by rising awareness of rare childhood cancers, improvements in diagnostic imaging and genetic testing, and increasing access to specialized pediatric oncology centers
- North America dominated the pleuropulmonary blastoma treatment market with the largest revenue share of 41.6% in 2025, supported by advanced healthcare infrastructure, strong research funding for rare diseases, and the presence of specialized children’s hospitals, with the U.S. witnessing consistent treatment adoption due to well-established clinical guidelines and collaborative research networks
- Asia-Pacific is expected to be the fastest growing region during the forecast period due to improving healthcare infrastructure, increasing investments in pediatric oncology, and rising awareness and diagnosis of rare pediatric cancers across emerging economies
- Chemotherapy segment dominated the pleuropulmonary blastoma treatment market with a market share of 46.2% in 2025, driven by its central role in multimodal treatment regimens and its effectiveness in managing advanced and recurrent cases
Report Scope and Pleuropulmonary Blastoma Treatment Market Segmentation
|
Attributes |
Pleuropulmonary Blastoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Pleuropulmonary Blastoma Treatment Market Trends
Advancements in Multimodal and Precision-Based Pediatric Oncology Care
- A significant and accelerating trend in the global pleuropulmonary blastoma treatment market is the growing adoption of multimodal treatment approaches that combine surgery, chemotherapy, and supportive care to improve survival outcomes in pediatric patients
- For instance, treatment protocols guided by international pediatric oncology groups increasingly emphasize early surgical resection followed by risk-adapted chemotherapy to reduce recurrence rates and disease progression
- Advancements in molecular diagnostics and genetic profiling, particularly related to DICER1 mutations, are enabling more precise risk stratification and personalized treatment planning for affected children, thereby improving clinical decision-making
- The integration of advanced imaging technologies and standardized clinical pathways across specialized pediatric oncology centers is facilitating more accurate diagnosis, staging, and monitoring of treatment response throughout the care continuum
- The increasing use of centralized rare disease registries is improving data sharing, longitudinal patient tracking, and evidence generation for optimizing treatment strategies in this ultra-rare cancer
- This trend toward more personalized, protocol-driven, and collaborative treatment strategies is reshaping clinical expectations in rare pediatric cancers, encouraging consistent adoption of evidence-based care practices
- The demand for comprehensive pleuropulmonary blastoma treatment solutions is steadily increasing as healthcare systems prioritize early intervention, multidisciplinary care teams, and improved long-term survival outcomes for rare pediatric malignancies
Pleuropulmonary Blastoma Treatment Market Dynamics
Driver
Rising Awareness of Rare Pediatric Cancers and Improving Access to Specialized Care
- The increasing awareness of rare pediatric cancers among healthcare professionals and caregivers, coupled with improving access to specialized pediatric oncology services, is a key driver supporting the demand for pleuropulmonary blastoma treatment
- For instance, growing participation in international rare cancer registries and pediatric oncology research networks is improving early diagnosis rates and standardizing treatment approaches across regions
- As diagnostic capabilities improve and awareness initiatives expand, more cases of pleuropulmonary blastoma are being identified at earlier stages, enabling timely intervention and better clinical outcomes
- Furthermore, the expansion of pediatric oncology centers and referral networks is strengthening access to advanced treatment options, particularly in developed healthcare systems
- Increasing public and private funding for rare pediatric disease research is supporting clinical studies, protocol refinement, and wider dissemination of treatment guidelines
- The involvement of multidisciplinary care teams, including pediatric surgeons, oncologists, genetic counselors, and pulmonologists, is enhancing treatment coordination and driving consistent therapy adoption
- The availability of structured treatment protocols, improved supportive care, and multidisciplinary expertise is encouraging consistent adoption of established therapies, thereby driving overall market growth
Restraint/Challenge
Limited Disease Awareness and Complexity of Treatment Standardization
- The rarity of pleuropulmonary blastoma and limited awareness among general healthcare providers pose a significant challenge to early diagnosis and timely treatment initiation, particularly in resource-limited settings
- For instance, delayed recognition of symptoms in non-specialized facilities can lead to advanced-stage diagnosis, negatively impacting treatment outcomes and survival rates
- Variability in access to specialized pediatric oncology centers across regions creates disparities in treatment quality and clinical outcomes
- The absence of widely approved targeted therapies limits treatment options and places continued reliance on intensive chemotherapy and surgical interventions
- While advancements in treatment protocols are improving outcomes, the complexity and intensity of multimodal therapies can strain healthcare resources and increase the overall cost burden for families and healthcare systems
- Managing long-term treatment-related complications, including pulmonary dysfunction and developmental impacts, remains a challenge in survivorship care
- Overcoming these challenges through improved awareness programs, broader access to specialized care, and strengthened international collaboration will be critical for sustaining growth in the pleuropulmonary blastoma treatment market
Pleuropulmonary Blastoma Treatment Market Scope
The market is segmented on the basis of disease type, treatment, diagnosis, dosage, route of administration, end-users, and distribution channel.
- By Disease Type
On the basis of disease type, the pleuropulmonary blastoma treatment market is segmented into Type I tumors, Type II tumors, and Type III tumors. The Type II tumors segment dominated the market with the largest revenue share in 2025, driven by its higher clinical severity and frequent need for aggressive multimodal treatment. Type II tumors are partially cystic and partially solid, often requiring a combination of surgery and chemotherapy. Patients diagnosed at this stage typically undergo prolonged therapy and close monitoring, contributing to increased healthcare utilization. The complexity of managing intermediate-stage disease also drives demand for specialized pediatric oncology services. In addition, Type II tumors often progress to more advanced forms if untreated, reinforcing early and intensive intervention. Hospitals and specialized centers prioritize Type II cases due to the intensive resources required.
The Type I tumors segment is expected to witness the fastest growth during the forecast period, supported by rising awareness and improved early diagnostic capabilities. Type I tumors are purely cystic and increasingly detected at earlier stages through prenatal imaging and pediatric screening. Early diagnosis enables timely surgical intervention, reducing disease progression and improving survival outcomes. Growing adoption of genetic testing for DICER1 mutations is also supporting earlier identification. Type I tumors require fewer intensive therapies, making them manageable in smaller clinics. As awareness improves globally, diagnosis rates of Type I tumors are expected to rise, accelerating segment growth.
- By Treatment
On the basis of treatment, the market is segmented into medication, chemotherapy, surgery, and radiation therapy. The chemotherapy segment dominated the market in 2025 with a market share of 46.2%, driven by its central role in treating intermediate and advanced pleuropulmonary blastoma cases. Chemotherapy is commonly used alongside surgery to reduce tumor burden and prevent recurrence, particularly in Type II and Type III tumors. The prolonged treatment cycles and use of multiple drug regimens significantly contribute to higher revenue generation. Chemotherapy remains the backbone of standardized treatment protocols recommended by pediatric oncology networks. Its proven effectiveness in improving survival rates further reinforces its dominant position. Hospitals and pediatric oncology centers rely on chemotherapy as a core treatment modality due to its efficacy.
The surgery segment is anticipated to be the fastest growing during the forecast period, owing to increasing emphasis on early-stage tumor resection. Surgical intervention is the primary treatment for Type I tumors and plays a critical role in disease management when detected early. Advances in pediatric thoracic surgery and minimally invasive techniques are improving outcomes and reducing recovery time. Early surgical removal can significantly lower the need for intensive chemotherapy, making it an attractive treatment approach. Rising awareness and screening programs are supporting higher surgical adoption. Surgery also allows precise tumor removal, reducing recurrence risk and improving prognosis.
- By Diagnosis
On the basis of diagnosis, the market is segmented into chest X-ray, CT scan, PET scan, MRI, bone scan, bronchoscopy, and thoracoscopy. The CT scan segment dominated the market in 2025, driven by its widespread use as the primary diagnostic imaging modality for pleuropulmonary blastoma. CT scans provide detailed visualization of lung structures, enabling accurate tumor detection, classification, and staging. They are routinely used in both initial diagnosis and treatment monitoring. High availability across hospitals and pediatric oncology centers further supports dominance. CT imaging is critical for surgical planning and chemotherapy evaluation. Its speed, resolution, and accessibility make it the preferred first-line imaging option.
The MRI segment is expected to register the fastest growth during the forecast period, supported by its superior soft-tissue contrast and absence of ionizing radiation. MRI is increasingly preferred for follow-up imaging in pediatric patients to minimize radiation exposure. It is particularly useful in assessing tumor spread and treatment response. Growing emphasis on safer diagnostic alternatives for children is accelerating MRI adoption. Technological advancements are also improving scan speed and accessibility. MRI allows repeated imaging without radiation risk, making it ideal for long-term monitoring.
- By Dosage
On the basis of dosage, the market is segmented into solution, powder, injection, and others. The injection segment dominated the market in 2025, primarily due to the intravenous administration of chemotherapy drugs. Injectable formulations ensure precise dosing and rapid systemic delivery, which is essential in pediatric oncology treatment. Most standard chemotherapy regimens rely on injectable drugs administered under controlled clinical settings. The need for hospital-based administration further supports higher utilization. Injectable dosage forms are critical for intermediate and advanced tumor stages. They also allow flexible regimen adjustments based on patient response.
The solution segment is expected to grow at the fastest rate during the forecast period, driven by increasing use in supportive care and adjunct therapies. Liquid formulations are easier to administer in pediatric patients and allow flexible dosing adjustments. Solutions are commonly used for pain management, hydration, and symptom control. Improved formulation stability and palatability are further encouraging adoption. Solutions reduce the need for hospital visits for minor interventions. Their convenience and ease of use make them increasingly preferred by caregivers and outpatient clinics.
- By Route of Administration
On the basis of route of administration, the market is segmented into intravenous and oral. The intravenous segment dominated the market in 2025, driven by the reliance on IV chemotherapy and hospital-based treatment protocols. Intravenous delivery ensures immediate bioavailability and controlled dosing, which is critical in aggressive cancer treatment. Most frontline therapies for pleuropulmonary blastoma are administered intravenously. The need for close monitoring during treatment further supports dominance. IV administration remains the clinical standard for severe cases. Hospitals rely on IV administration to deliver precise and potent chemotherapy doses safely.
The oral segment is expected to witness the fastest growth during the forecast period, supported by increasing use of oral supportive medications and follow-up therapies. Oral administration offers convenience and improves treatment compliance, particularly during long-term care. It reduces hospitalization burden and supports home-based management where appropriate. Advances in pediatric oral formulations are further enabling growth. Oral medications facilitate adherence for outpatient therapy. Families prefer oral drugs for convenience and reduced clinical visits.
- By End-Users
On the basis of end-users, the market is segmented into clinics, hospitals, and others. The hospital segment dominated the market in 2025, driven by the complexity of pleuropulmonary blastoma treatment. Hospitals offer integrated oncology services, including diagnostics, surgery, chemotherapy, and intensive care. Specialized pediatric oncology hospitals play a central role in disease management. The need for multidisciplinary teams and advanced infrastructure supports high hospital utilization. Most treatment protocols are executed in hospital settings. Hospitals also provide post-treatment monitoring and supportive care, which increases segment revenue.
The clinic segment is expected to grow at the fastest rate during the forecast period, owing to increasing outpatient follow-up and supportive care services. Clinics are increasingly used for monitoring, rehabilitation, and long-term survivorship care. Improved referral networks are enabling clinics to manage non-acute phases of treatment. This shift helps reduce hospital burden while maintaining continuity of care. Expansion of specialty pediatric clinics is supporting growth. Clinics also provide easier access for families in semi-urban and regional areas.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market in 2025, supported by the hospital-centric nature of chemotherapy and injectable drug administration. Hospital pharmacies ensure controlled storage, preparation, and dispensing of oncology drugs. They are integral to treatment safety and protocol adherence. Most high-cost and specialized medications are dispensed directly through hospital systems. This centralization supports revenue dominance. Hospital pharmacies also manage bulk procurement for inpatient therapy, adding to market share.
The online pharmacy segment is expected to be the fastest growing during the forecast period, driven by increasing demand for supportive medications and follow-up drugs. Online platforms offer convenience and improved access, particularly for long-term care needs. Growth in digital health adoption and e-pharmacy regulations is supporting expansion. Families increasingly prefer home delivery for non-critical medications. The rise of telemedicine and virtual consultations is boosting online pharmacy adoption. Online channels improve medication adherence and accessibility, especially in remote areas.
Pleuropulmonary Blastoma Treatment Market Regional Analysis
- North America dominated the pleuropulmonary blastoma treatment market with the largest revenue share of 41.6% in 2025, supported by advanced healthcare infrastructure, strong research funding for rare diseases, and the presence of specialized children’s hospitals
- Patients in the region benefit from access to specialized children’s hospitals, well-established treatment protocols, and multidisciplinary care teams that provide integrated surgical, chemotherapy, and supportive care
- This widespread adoption is further supported by strong research funding, early diagnostic capabilities including genetic testing for DICER1 mutations, and high public awareness of rare pediatric cancers, establishing North America as a leading market for pleuropulmonary blastoma treatment services
U.S. Pleuropulmonary Blastoma Treatment Market Insight
The U.S. pleuropulmonary blastoma treatment market captured the largest revenue share of 82% in 2025 within North America, fueled by advanced pediatric oncology infrastructure and early diagnosis capabilities. Patients benefit from access to specialized children’s hospitals, multidisciplinary care teams, and integrated treatment protocols including surgery and chemotherapy. The growing awareness of rare childhood cancers, coupled with widespread availability of genetic testing for DICER1 mutations, is increasing early intervention rates. Furthermore, the presence of established pediatric oncology research networks and clinical trial programs is accelerating the adoption of novel treatment strategies. Rising public and private funding for rare pediatric cancers further supports market expansion. These factors collectively establish the U.S. as the dominant market for pleuropulmonary blastoma treatment in North America.
Europe Pleuropulmonary Blastoma Treatment Market Insight
The Europe pleuropulmonary blastoma treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by improved access to pediatric oncology services and rare disease initiatives. Countries are increasingly focusing on early diagnosis through imaging and genetic testing, and treatment adoption is rising in both public and private healthcare facilities. The growing urban population and rising awareness of rare pediatric cancers are encouraging investment in specialized treatment centers. European governments and NGOs are also promoting collaborative clinical research and cross-border pediatric oncology networks. Furthermore, standardized treatment guidelines and protocol-based therapies are increasing the effectiveness and accessibility of pleuropulmonary blastoma care across the region. The adoption of advanced multimodal therapies is becoming prevalent in both hospitals and specialized clinics.
U.K. Pleuropulmonary Blastoma Treatment Market Insight
The U.K. pleuropulmonary blastoma treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing awareness of rare pediatric cancers and access to specialized oncology centers. The country emphasizes early detection, multidisciplinary treatment teams, and risk-adapted chemotherapy protocols, ensuring timely intervention. The NHS and private hospitals are integrating advanced diagnostic techniques, including CT, MRI, and genetic testing, to improve patient outcomes. Public campaigns and pediatric oncology registries are raising awareness among caregivers and healthcare providers. Growing demand for comprehensive treatment services in hospitals and outpatient clinics is supporting market expansion. Furthermore, the focus on survivorship care and long-term follow-up is enhancing overall adoption of pleuropulmonary blastoma therapies.
Germany Pleuropulmonary Blastoma Treatment Market Insight
The Germany pleuropulmonary blastoma treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by rising awareness of rare childhood cancers and robust healthcare infrastructure. Advanced pediatric oncology hospitals are offering integrated treatment solutions, including surgery, chemotherapy, and supportive care. Emphasis on innovation and clinical research in rare pediatric cancers is promoting adoption of new treatment protocols. Germany’s well-established healthcare reimbursement system improves access to high-cost therapies. Multidisciplinary teams and modern diagnostic capabilities, including imaging and genetic profiling, support precise treatment planning. The focus on evidence-based care and standardized protocols ensures consistent treatment outcomes across the country.
Asia-Pacific Pleuropulmonary Blastoma Treatment Market Insight
The Asia-Pacific pleuropulmonary blastoma treatment market is poised to grow at the fastest CAGR of 25% during the forecast period of 2026 to 2033, driven by improving healthcare infrastructure and rising awareness of rare pediatric cancers in countries such as China, Japan, and India. Increasing government initiatives promoting pediatric oncology services and early cancer detection are driving treatment adoption. Expanding specialized hospitals and clinics, along with investments in diagnostic imaging and genetic testing, are supporting the growth of multimodal therapy approaches. Furthermore, rising disposable incomes and growing urbanization are increasing access to advanced treatment options. Collaborations with international pediatric oncology networks are enhancing knowledge transfer and protocol standardization. As awareness spreads, the availability and adoption of pleuropulmonary blastoma treatments are expected to expand rapidly across APAC.
Japan Pleuropulmonary Blastoma Treatment Market Insight
The Japan pleuropulmonary blastoma treatment market is gaining momentum due to the country’s advanced healthcare system, high technological adoption, and emphasis on early detection of rare pediatric cancers. Multidisciplinary pediatric oncology centers provide integrated treatment, including surgery, chemotherapy, and long-term supportive care. Early genetic screening programs, particularly for DICER1 mutations, are improving diagnosis and risk stratification. Japan’s focus on precision medicine and clinical research promotes adoption of innovative treatment protocols. The aging population further increases the demand for efficient and safe pediatric care practices. Integration of advanced imaging and hospital-based follow-up care ensures better outcomes for affected children.
India Pleuropulmonary Blastoma Treatment Market Insight
The India pleuropulmonary blastoma treatment market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to improving healthcare infrastructure, rising awareness of rare childhood cancers, and increasing availability of specialized pediatric oncology centers. The country’s growing middle class and urbanization are driving adoption of early diagnosis and multimodal treatment strategies. Government initiatives supporting pediatric cancer care, along with private hospital networks, are expanding treatment access. Affordable treatment options, coupled with increased training of pediatric oncology specialists, are contributing to market growth. The rising number of diagnostic imaging facilities and genetic testing labs is further facilitating timely detection and intervention. These factors are establishing India as a key growth hub for pleuropulmonary blastoma treatment in the region.
Pleuropulmonary Blastoma Treatment Market Share
The Pleuropulmonary Blastoma Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- AstraZeneca (U.K.)
- Novartis AG (Switzerland)
- Merck & Co., Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Enzo Biochem Inc. (U.S.)
- Zuche Pharmaceuticals (India)
- Helix biopharma (Canada)
- Bristol Myers Squibb Company (U.S.)
- F. Hoffmann La Roche Ltd (Switzerland)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
- GSK plc (U.K.)
- Amgen Inc. (U.S.)
- AbbVie Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Blueprint Medicines Corporation (U.S.)
- BridgeBio Pharma, Inc. (U.S.)
What are the Recent Developments in Global Pleuropulmonary Blastoma Treatment Market?
- In August 2025, the Phase III clinical trial ARAR2331 began active recruitment to evaluate prospective treatment strategies including surgery with or without chemotherapy for Types I, II, and III PPB, marking one of the first large-scale collaborative interventional studies aimed at refining standardized care pathways
- In April 2025, the National Cancer Institute’s pediatric treatment summary for pleuropulmonary blastoma (PPB) was updated to include new screening insights from the International PPB/DICER1 Registry, noting cystic lesion identification and resection patterns in individuals with germline DICER1 variants reflecting evolving clinical surveillance practices for early disease detection
- In March 2025, a case series on PPB with brain metastasis highlighted multidisciplinary treatment approaches involving surgery, chemotherapy, and radiotherapy for children with PPB metastatic to the central nervous system, underscoring evolving clinical management strategies for advanced presentations of the disease
- In October 2024, a multicenter clinical study analyzing four pediatric PPB cases was published, offering fresh data on clinical characteristics and outcomes that contribute to global understanding of disease presentation and treatment responses in real-world settings
- In October 2024, a multicase clinical analysis of pediatric pleuropulmonary blastoma was published in BMC Cancer, reporting detailed clinical characteristics and treatment outcomes from four PPB cases, highlighting surgical resection and chemotherapy approaches in real-world clinical settings and reinforcing the importance of multimodal management in improving diagnostic and therapeutic strategies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.