Global Nucleic Acid Amplification Market
Market Size in USD Billion
CAGR :
% 
 USD
3.90 Billion
USD
7.95 Billion
2024
2032
USD
3.90 Billion
USD
7.95 Billion
2024
2032
| 2025 –2032 | |
| USD 3.90 Billion | |
| USD 7.95 Billion | |
|
|
|
|
Nucleic Acid Amplification Market Size
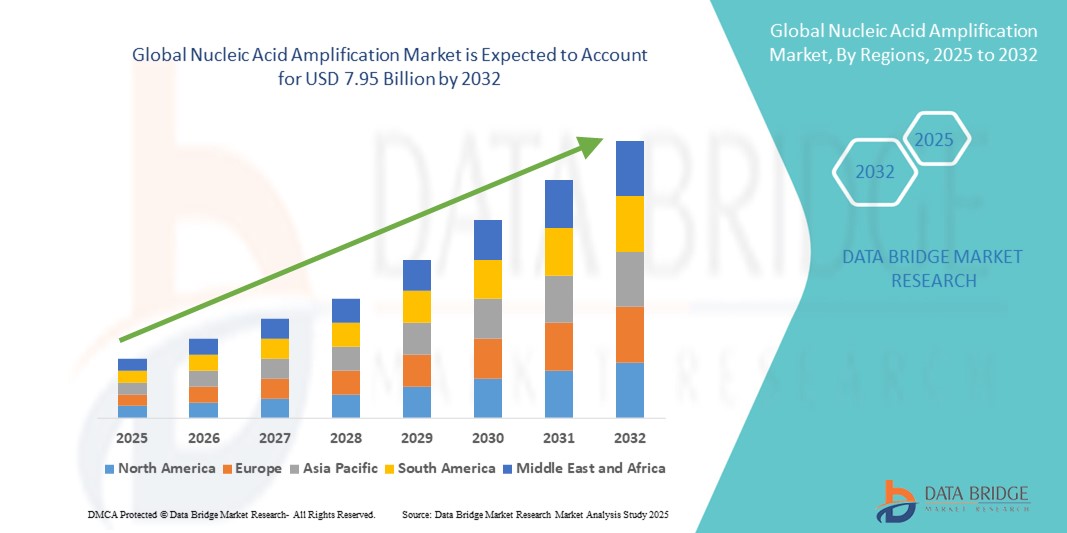
- The global nucleic acid amplification market size was valued at USD 3.90 billion in 2024 and is expected to reach USD 7.95 billion by 2032, at a CAGR of 9.30% during the forecast period
- The market growth is primarily driven by increasing demand for rapid, sensitive, and accurate molecular diagnostic techniques across healthcare, research, and forensic applications
- In addition, advancements in amplification technologies, rising prevalence of infectious diseases, and expanding applications in personalized medicine are fueling adoption worldwide. These factors collectively are accelerating the integration of nucleic acid amplification methods, thereby significantly propelling the market’s expansion
Nucleic Acid Amplification Market Analysis
- Nucleic acid amplification technologies provide rapid and precise detection of genetic material, becoming essential tools in molecular diagnostics, infectious disease testing, and genetic research within clinical and laboratory environments
- The growing demand is largely driven by the increasing prevalence of infectious diseases, advancements in amplification techniques such as PCR and isothermal amplification, and rising adoption of molecular diagnostics for personalized medicine and cancer detection
- North America dominated the nucleic acid amplification market with the largest revenue share of 39.4% in 2024, supported by advanced healthcare infrastructure, high R&D investments, and presence of key players innovating in diagnostic assays and platforms
- Asia-Pacific is expected to be the fastest-growing region in the nucleic acid amplification market during the forecast period due to expanding healthcare access, increasing infectious disease burden, and rising government initiatives promoting molecular diagnostics
- Infectious diseases segment dominated the nucleic acid amplification market with a market share of 52.5% in 2024, driven by urgent demand for rapid pathogen detection and outbreak management in clinical settings
Report Scope and Nucleic Acid Amplification Market Segmentation
|
Attributes |
Nucleic Acid Amplification Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Nucleic Acid Amplification Market Trends
Advancements in Rapid and Point-of-Care Testing Technologies
- A major and accelerating trend in the global nucleic acid amplification market is the development and adoption of rapid, portable point-of-care (POC) diagnostic devices that provide timely results outside traditional laboratory settings
- For instance, platforms such as Cepheid’s GeneXpert and Abbott’s ID NOW enable quick detection of infectious agents such as COVID-19 and influenza, improving patient outcomes through faster clinical decision-making
- Innovations in isothermal amplification techniques such as LAMP (Loop-mediated Isothermal Amplification) allow amplification without complex thermal cycling, making devices more accessible for decentralized testing and resource-limited environments
- Integration with digital health platforms and cloud-based data management systems is enhancing result reporting, remote monitoring, and epidemiological surveillance capabilities
- This trend is transforming molecular diagnostics by enabling decentralized testing, reducing turnaround times, and increasing accessibility in both developed and emerging markets
- Companies such as BioFire Diagnostics and Qiagen are actively advancing portable nucleic acid amplification solutions with multiplex testing capabilities and user-friendly interfaces, addressing the growing demand for rapid, on-site diagnostics across healthcare and public health sectors
Nucleic Acid Amplification Market Dynamics
Driver
Increasing Demand Due to Rising Infectious Diseases and Personalized Medicine
- The rising prevalence of infectious diseases globally, including emerging viral outbreaks and antibiotic-resistant bacterial infections, is a significant driver for nucleic acid amplification technologies that offer sensitive and specific pathogen detection
- For instance, in 2024, Roche Diagnostics expanded its molecular testing portfolio with new assays targeting infectious agents, enabling quicker identification and management of diseases
- Growing adoption of personalized medicine and genetic testing for cancer and rare diseases further propels demand for precise nucleic acid amplification methods that support targeted therapies and diagnostics
- Increasing investments in healthcare infrastructure, especially in developing countries, and government initiatives to enhance diagnostic capabilities contribute to market growth
- The need for rapid, accurate, and cost-effective molecular diagnostics across clinical, research, and forensic applications continues to fuel the market’s expansion
Restraint/Challenge
Technical Complexity and Regulatory Hurdles
- The complexity of nucleic acid amplification technologies and stringent regulatory requirements for diagnostic assay approval present challenges to market growth and product commercialization
- High costs associated with advanced equipment, reagents, and skilled personnel can limit adoption, particularly in low-resource settings
- For instance, in 2023, a leading diagnostic company faced delays in FDA approval for a novel PCR-based assay due to stringent validation requirements, impacting its market entry timeline
- In addition, issues related to contamination risks, assay sensitivity, and reproducibility require continuous quality control and validation, adding operational burdens for laboratories
- Overcoming these challenges through technological simplification, cost reduction, streamlined regulatory pathways, and robust quality assurance will be essential for broader market penetration and sustained growth
Nucleic Acid Amplification Market Scope
The market is segmented on the basis of application and technique.
- By Type
On the basis of application, the nucleic acid amplification market is segmented into infectious diseases, cancer, personalized medicine, and genetic testing. The infectious diseases segment dominated the market with the largest revenue share of 52.5% in 2024, driven by the critical need for rapid and accurate pathogen detection in clinical diagnostics and outbreak management. The high prevalence of infectious diseases worldwide and ongoing viral outbreaks are key factors sustaining strong demand in this segment.
The cancer segment is expected to witness the fastest CAGR from 2025 to 2032, supported by the rising adoption of molecular diagnostics for early cancer detection, mutation analysis, and therapy monitoring. Personalized medicine is also growing rapidly due to increasing focus on targeted treatments based on genetic profiles. Genetic testing is expanding steadily, driven by applications in hereditary disease screening and research.
- By Technique
On the basis of technique, the nucleic acid amplification market is segmented into target amplification, probe amplification, and signal amplification. The target amplification segment dominated the market with a revenue share of approximately 57% in 2024, mainly due to widespread use of PCR and related methods that amplify specific DNA or RNA sequences for sensitive and specific detection.
The probe amplification segment is expected to witness the fastest CAGR from 2025 to 2032, as it enhances detection specificity by amplifying the probe signal rather than the target sequence, useful in certain diagnostic applications requiring high accuracy. Signal amplification is expected to witness increasing adoption due to its ability to improve assay sensitivity without amplifying the target nucleic acid itself, beneficial in complex sample matrices and multiplex testing.
Nucleic Acid Amplification Market Regional Analysis
- North America dominated the nucleic acid amplification market with the largest revenue share of 39.4% in 2024, supported by advanced healthcare infrastructure, high R&D investments, and presence of key players innovating in diagnostic assays and platforms
- Healthcare providers and research institutions in the region prioritize rapid, accurate diagnostics for infectious diseases, cancer, and genetic disorders, supported by extensive R&D activities and presence of key industry players
- The region’s well-established regulatory framework and growing emphasis on personalized medicine further accelerate the uptake of nucleic acid amplification assays, making North America a leading market for innovative molecular diagnostic solutions in both clinical and research settings
U.S. Nucleic Acid Amplification Market Insight
The U.S. nucleic acid amplification market captured the largest revenue share of 39% in 2024 within North America, driven by the rapid adoption of advanced molecular diagnostic technologies and growing demand for quick, accurate infectious disease testing. The expanding use of point-of-care platforms, combined with increased investments in healthcare R&D and digital integration, further propels market growth. Moreover, the integration of nucleic acid amplification assays with AI-powered data analytics and electronic health records is significantly enhancing diagnostic efficiency and outcomes.
Europe Nucleic Acid Amplification Market Insight
The Europe nucleic acid amplification market is expected to grow steadily throughout the forecast period, driven by stringent regulatory standards, increasing prevalence of infectious diseases, and rising demand for early cancer detection and genetic testing. Countries such as Germany and the U.K. show strong market growth supported by increasing government funding for molecular diagnostics and rising healthcare digitization. The region emphasizes high-quality diagnostics and personalized medicine, which fuels the adoption of nucleic acid amplification technologies across clinical and research institutions.
U.K. Nucleic Acid Amplification Market Insight
The U.K. nucleic acid amplification market is anticipated to expand at a notable CAGR during the forecast period, supported by rising investments in genomic research and growing implementation of precision medicine. Public health initiatives targeting infectious disease control and cancer screening are driving demand for nucleic acid amplification assays. The country’s robust biotechnology sector and strong healthcare infrastructure further stimulate growth, with increasing incorporation of point-of-care molecular diagnostics in hospitals and clinics.
Germany Nucleic Acid Amplification Market Insight
The Germany nucleic acid amplification market is projected to experience significant growth, fueled by strong emphasis on healthcare innovation and digital transformation. The country prioritizes early disease diagnosis and preventive healthcare, encouraging adoption of advanced nucleic acid amplification technologies. In addition, stringent data privacy and security regulations lead to demand for secure and reliable diagnostic solutions. Integration with automated laboratory systems and smart data management platforms is becoming more prevalent in Germany’s clinical laboratories.
Asia-Pacific Nucleic Acid Amplification Market Insight
The Asia-Pacific nucleic acid amplification market is poised to grow at the fastest CAGR of around 22% during 2025 to 2032, driven by rising healthcare expenditure, increasing infectious disease burden, and expanding molecular diagnostics adoption in countries such as China, India, and Japan. Government initiatives promoting digital healthcare, growing awareness about personalized medicine, and expanding biotechnology infrastructure are accelerating market growth. In addition, the region benefits from local manufacturing of reagents and instruments, improving affordability and accessibility of nucleic acid amplification solutions.
Japan Nucleic Acid Amplification Market Insight
The Japan nucleic acid amplification market growth is supported by the country’s advanced healthcare system, aging population, and strong focus on technological innovation. The rising prevalence of chronic diseases and increasing use of molecular diagnostics in cancer and genetic disorder management boost demand. Integration of nucleic acid amplification with IoT and AI-driven health monitoring systems is also enhancing the efficiency and accuracy of diagnostics in Japan.
India Nucleic Acid Amplification Market Insight
The India nucleic acid amplification market accounted for the largest revenue share within Asia-Pacific in 2024, propelled by rapid urbanization, expanding healthcare infrastructure, and increasing government focus on infectious disease control. The growing middle-class population and rising awareness of molecular diagnostics are fueling adoption in both public and private healthcare sectors. The push for affordable diagnostic solutions and growing domestic production capabilities are further accelerating market growth in India.
Nucleic Acid Amplification Market Share
The Nucleic Acid Amplification industry is primarily led by well-established companies, including:
- Siemens (Germany)
- Hologic, Inc. (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Abbott (U.S.)
- BD (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Thermo Fisher Scientific Inc. (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- NeuroLogica Corp. (U.S.)
- Shimadzu Medical (India) pvt. Ltd. (Japan)
- General Electric (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Sysmex India Pvt. Ltd. (Japan)
- Hitachi, Ltd. (Japan)
- Canon Inc. (Japan)
- FUJIFILM Holdings Corporation (U.K.)
- Illumina, Inc. (U.S.)
- Danaher Corporation (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Novartis AG (Switzerland)
- Seegene Inc. (South Korea)
What are the Recent Developments in Global Nucleic Acid Amplification Market?
- In June 2025, bioMérieux SA published an automated nucleic acid amplification assay for detecting mycoplasma in cell and gene therapy products, offering a high-throughput, in‑house solution based on isothermal technology, delivering results in about 1 hour with minimal technical effort
- In May 2025, QIAGEN N.V. signed a definitive agreement to acquire Genoox, Ltd. for USD 80 million (comprising USD 70 million in cash plus up to USD 10 million in contingent payments), aiming to enhance its clinical genomics and molecular diagnostics offerings
- In May 2025, A newly published study demonstrates a reverse transcription recombinase polymerase amplification (RT‑RPA) assay coupled with a lateral flow detection (LFD) platform for rapid, sensitive, and specific detection of dengue virus serotype 2 (DENV2). The assay achieves a limit of detection of approximately 50 copies per reaction, exhibits no cross-reactivity with other common pathogens, and performs reliably under variable environmental conditions
- In April 2025, Researchers published a PCR-based, open-source point-of-care platform that offers 100% sensitivity and over 98% specificity in detecting respiratory and HPV viruses, with ROC AUC values exceeding 0.98 marking it a highly accurate and low-cost alternative for decentralized diagnostics
- In April 2025, Meridian Bioscience, Inc. introduced new enzyme stabilization services for molecular diagnostics encompassing qPCR, isothermal amplification, and NGS—designed to improve reagent stability, enable ambient-temperature formats, streamline workflows, and reduce costs
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.