Global Non Invasive Prenatal Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
4.75 Billion
USD
13.35 Billion
2024
2032
USD
4.75 Billion
USD
13.35 Billion
2024
2032
| 2025 –2032 | |
| USD 4.75 Billion | |
| USD 13.35 Billion | |
|
|
|
|
Non-invasive Prenatal Testing Market Analysis
After introducing next-generation sequencing technology, it is now possible to sequence foetal DNA fragments that can be built into a whole genetic map, allowing the foetal genome to be inspected prenatally and non-invasively. Novel screening approaches for foetal chromosomal aneuploidies have been developed as a result of advances in molecular technologies and the identification of cell-free foetal DNA in maternal plasma.
Non-invasive Prenatal Testing Market Size
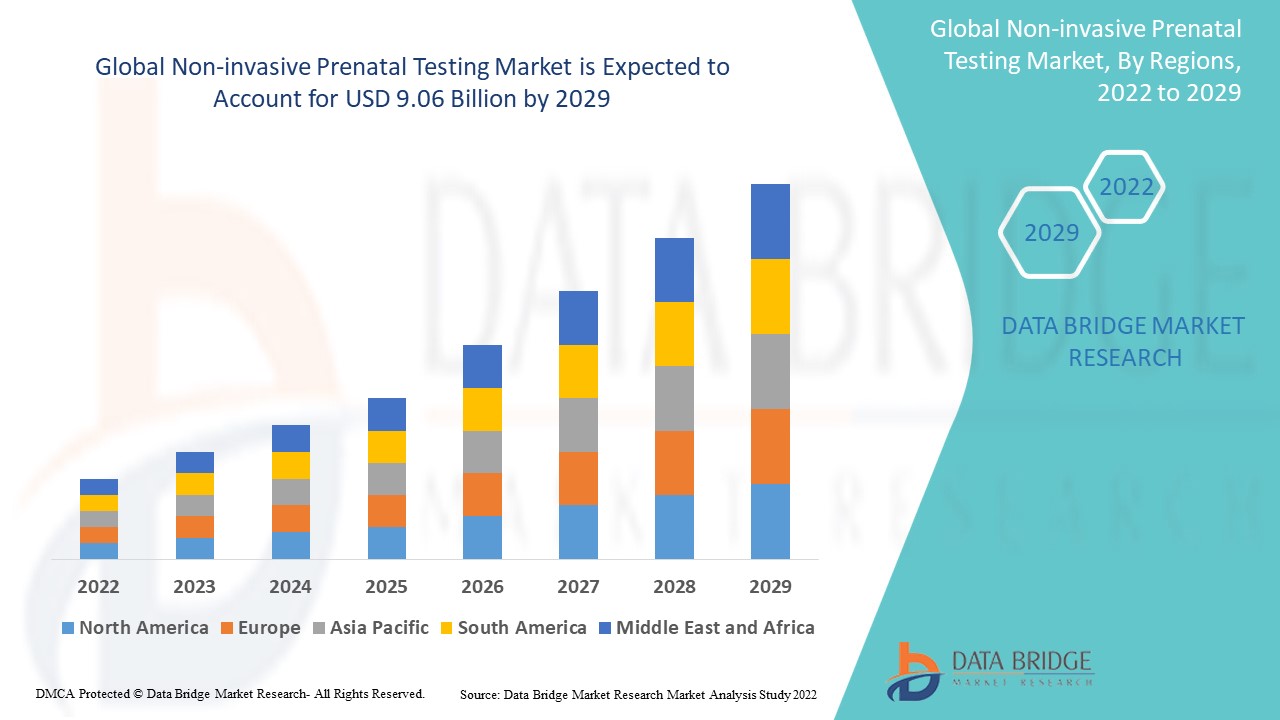
Global non-invasive prenatal testing market size was valued at USD 4.75 billion in 2024 and is projected to reach USD 13.35 billion by 2032, with a CAGR of 13.80% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Non-invasive Prenatal Testing Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Illumina, Inc. (U.S), Thermo Fisher Scientific Inc. (U.S), General Electric (U.S), BGI Group (China), Agilent Technologies, Inc. (U.S), F. Hoffmann-La Roche Ltd. (Switzerland), PerkinElmer Inc. (U.S), Laboratory Corporation of America Holdings (U.S), Natera, Inc. (U.S), Yourgene Health (U.S), Eurofins LifeCodexx GmbH (Germany), Quest Diagnostics Incorporated (U.S), Myriad Genetics Inc. (U.S), NIPD Genetics (Cyprus), Next Biosciences (South Africa) |
|
Market Opportunities |
|
Non-invasive Prenatal Testing Market Definition
Non-invasive prenatal testing is a DNA-based blood test that is used to assess the risk of genetic disorders in the developing foetus. This is done to see if you have Down syndrome, Patau syndrome, or Edwards syndrome, among other things.
Non-invasive Prenatal Testing Market Dynamics
Drivers
- High risk of chromosomal abnormalities with increasing maternal age
The age of a woman at the time of delivery is referred to as her maternal age. Increasing maternal age can lead to a variety of health issues, including reduced fertility, high blood pressure, and an increased chance of miscarriage, stillbirths, and maternal mortality. Furthermore, as a result of faulty chromosomal division, maternal age can raise the incidence of genetic abnormalities in newborns. Microcephaly, a short neck, upward slanted eyes, weak muscular tone, and heart, intestinal, and breathing difficulties are all possible outcomes in neonates. These data imply that there has been an increase in maternal advanced age over the world, which is likely to lead to more health issues. Thus, the increasing maternal age, coupled with the increasing incidence of chromosomal abnormalities, is expected to drive the demand for NIPT tests.
- Growing incidence of chromosomal abnormalities
The rising frequency of chromosomal abnormalities, as well as increased product utilisation in new applications, are driving the market's organic revenue growth. Furthermore, innovations in existing tests in terms of expanded functionality, improved chemistry, and bioinformatics analysis are projected to drive market growth. The improvement of payment policies for ordinary and low-risk pregnancies is one of the market's primary driving forces. Natera announced in December 2020 that its non-invasive prenatal testing (NIPT) would be covered for all pregnancies by Aetna, the leading U.S. health insurer.
- High incidence rate of down syndrome
One of the key factors driving the market for non-invasive prenatal testing (NIPT) is the rise in average maternal age, which has raised concerns about the fetus's safety. Down syndrome is a chromosomal disorder in which an extra chromosome 21 is formed as a result of a cell division error, and it can damage a foetus' cognitive abilities and physical growth. The NIPT screening tests are safe for both the mother and the baby, pose no risk of miscarriage, and can detect more than 99 percent of Down syndrome instances. According to the National Down Syndrome Society and the Centers for Disease Control and Prevention, one out of every 700 babies in the United States is born with Down syndrome, and 80 percent of Down syndrome children are born to older moms. The increased risk of having babies with Down syndrome among older women drives the non-invasive prenatal testing market.
Opportunities
NIPT can be used to detect chromosomal abnormalities such as Down syndrome, trisomy 18 and 13, as well as excess or missing X or Y chromosomes. The likelihood of a genetic disease in the foetus is determined via non-invasive prenatal examinations. The market for non-invasive prenatal testing is being driven by an increase in the number of kids with chromosomal disorders and improved diagnostic imaging technology. Growing insurance coverage for average and low-risk pregnancies is also expected to have a benefit the non-invasive prenatal testing market.
Restraints/Challenges
- Reliability of test results, especially in obese women
NIPT tests are available to patients between the ages of 10 and 20 weeks of pregnancy. These tests need the extraction of foetal DNA from maternal blood in order to determine chromosomal abnormalities. The mother makes up the majority of cell-free DNA circulating in maternal blood, with only 10-15 percent coming from the foetus. The amount of foetal fraction in the blood is influenced by a variety of circumstances. Maternal weight, for instance, has a significant impact on the amount of foetal fraction in maternal blood. Low quantities of foetal DNA in the plasma of obese women can lead to erroneous results when testing for trisomy and other disorders.
Patients who weigh more than 250 pounds may experience unclear outcomes as a result, NIPT cannot detect chromosomal abnormalities in obese women. Furthermore, positive NIPT results may demand invasive confirmation tests such as amniocentesis and CVS. This poses a significant threat to the non-invasive prenatal testing market expansion.
This non-invasive prenatal testing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the non-invasive prenatal testing market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Non-invasive Prenatal Testing Market Scope
The non-invasive prenatal testing market is segmented on the basis of product, method, application, test type, technology and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Products
- Consumables
- Assay Kits and Reagents
- Disposables
- Instruments
- NGS Systems
- PCR Instruments
- Microarrays
- Ultrasound Devices
- Other Instruments
- Services
Method
- Ultrasound Detection
- Biochemical Screening Tests
- Cell-free DNA in Maternal Plasma Tests
Application
- Trisomy
- Microdeletion Syndrome
- Other Applications
Test Type
- Materni21
- Harmony
- Panorama
- Verify
- NIFTY
- Others
Technology
- NGS
- WGS
- Others
End-User
- Diagnostic Laboratories
- Hospitals
Non-invasive Prenatal Testing Market Regional Analysis
The non-invasive prenatal testing market is analyzed and market size insights and trends are provided by country, product, method, application, test type, technology and end-user as referenced above.
The countries covered in the non-invasive prenatal testing market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the largest share for non-invasive prenatal testing market in the forecast period of 2025 to 2032.
Asia-Pacific is expected to have the highest CAGR due to development in the healthcare infrastructure and increasing awareness programs for NIPT.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Non-invasive Prenatal Testing Market Share
The non-invasive prenatal testing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to non-invasive prenatal testing market.
Non-invasive Prenatal Testing Market Leaders Operating in the Market Are:
- Illumina, Inc. (U.S)
- Thermo Fisher Scientific Inc. (U.S)
- General Electric (U.S)
- BGI Group (China)
- Agilent Technologies, Inc. (U.S)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- PerkinElmer Inc. (U.S)
- Laboratory Corporation of America Holdings (U.S)
- Natera, Inc. (U.S)
- Yourgene Health (U.S)
- Eurofins LifeCodexx GmbH (Germany)
- Quest Diagnostics Incorporated (U.S)
- Myriad Genetics Inc. (U.S)
- NIPD Genetics (Cyprus)
- Next Biosciences (South Africa)
Latest Developments in Non-invasive Prenatal Testing Market
- In January 2022, to deliver non-invasive prenatal testing (NIPT) solutions to QIAGEN's dPCR business, QIAGEN formed partnerships with Atila BioSystems
- In June 2021, Next-generation genomics and Illumina Thailand has launched the VeriSeq NIPT Solution v2, a next-generation sequencing (NGS)-based solution that helps detect anomalies missed by targeted tests
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.