Global Non-Invasive Brain Trauma Monitoring Devices Market, By Type (Non-invasive Intracranial Pressure Monitor, Non-invasive Cerebral Edema Dynamic Monitor, Others), By Product (Consumables and Monitoring Devices), Applications (Cardiology, Urology and Nephrology, Oncology, Gastroenterology, Others), End-User (Hospitals, Neurological Centers and Others) – Industry Trends and Forecast to 2029
Market Analysis and Size
Traumatic brain injury (TBI) is a serious condition that affects millions of people each year. For instance, according to a World Health Organization (WHO) study, brain damage is a major cause of disability and death worldwide. Most people with minor injuries do not need to be hospitalized. People with severe head injuries should be treated at a hospital or neuro center. The market for non-invasive brain trauma monitoring equipment is growing significantly due to increased awareness and improvement of healthcare facilities.
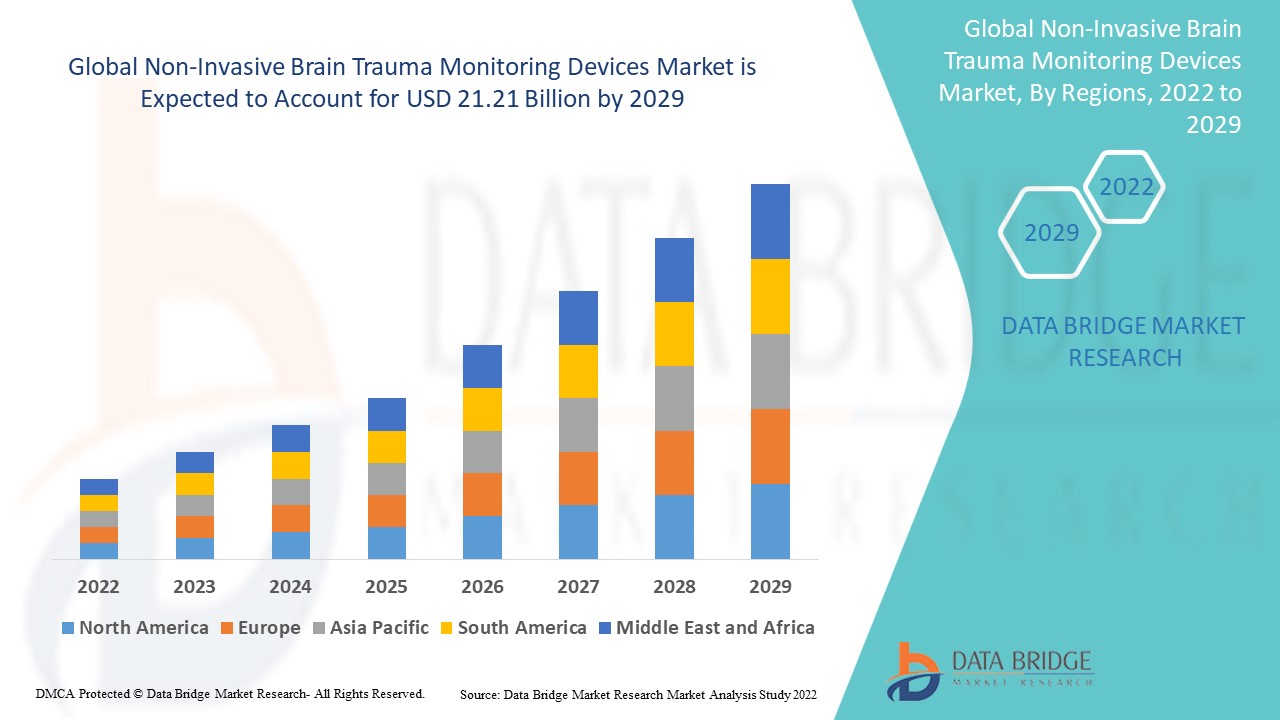
Data Bridge Market Research analyses a growth rate in the non-invasive brain trauma monitoring devices market in the forecast period 2022-2029. The expected CAGR of non-invasive brain trauma monitoring devices market is tend to be around 8.30% in the mentioned forecast period. The USD market value was found to be 11.21 billion in 2021and is expected to see a growth of 21.21 billion by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Definition
Non-invasive brain traumatic monitors are increasingly being used to monitor the progression of primary brain injury and limit secondary brain injury to patients in hospitals or neuro centers. In traumatic brain injury, the main advantage of a non-invasive brain injury monitoring device is its anti-inflammatory effect as it suppresses the release of inflammatory chemicals after head injury. Continuous monitoring of brain trauma in critical care patients gives physicians information about when and when to take action to reduce brain trauma.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014- 2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
By Type (Noninvasive Intracranial Pressure Monitor, Noninvasive Cerebral Edema Dynamic Monitor), By Product (Consumables and Monitoring Devices), Applications (Cardiology, Urology and Nephrology, Oncology, Gastroenterology, Others), End-User (Hospitals, Neurological Centers and Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
|
|
Market Players Covered
|
Advanced Brain Monitoring, Inc., (US), Cadwell Industries, Inc., (US), Canon Medical Systems Corporation (Japan), CAS Medical Systems, Inc. (US), Codman & Shurtleff, Inc(US), Compumedics Ltd.(Australia), Magstim EGI (US), General Electric Company(US), Hitachi, Ltd. (Japan), Koninklijke Philips N.V. (Netherlands), Medtronic (US), Natus Medical Incorporated (US), Neuro Logica Corp. (US), NeuroWave Systems, Inc.(US), Nihon Kohden Corporation(Japan), Noraxon U.S.A., Inc.(US), RAUMEDIC AG(Germany), Sense Neuro Diagnostics(US), Siemens(Germany), Sophysa Ltd. (US), Spiegelberg GmbH & Co. KG (Germany)
|
|
Market Opportunities
|
|
Global Non-Invasive Brain Trauma Monitoring Devices Market Dynamics
Drivers
- Increased Neurological Disorders Promote the Growth
With increasing prevalence of neurological damage and disease, the neurological device industry is growing significantly. Neurological devices can be intrinsically invasive, for instance, intracranial pressure monitor used in severe cases. Hence, all these neurological disorders are promoting the growth of the market.
- Feasibility of non-invasive brain trauma monitoring devices
A device for non-invasive monitoring of brain trauma allows neurologists to measure brain health without being invasive. Devices for non-invasive brain trauma monitoring help achieve results quickly and safely. In addition, they are easy to use and provide accurate results. They are less traumatic and allow the patient to recover quickly.
Opportunities
Technological Advances In the recent times, the more involvement of advanced technology is boosting the market growth rapidly. Companies are focused on manufacturing digital devices such as mobile devices and wireless technologies, which can be processed easily and quickly.
- Increased Research and Standardization of Procedures
Standardization of brain monitoring procedures and an increase in clinical trials for neurocognitive disorders are expected to provide good opportunities for the non-invasive brain damage monitoring device market.
Restraints/Challenges
- High cost of Non-Invasive Brain Trauma Monitoring Devices
The high cost of various non-invasive brain trauma monitoring devices is a major constraint on the market, especially in emerging countries. These factors have led to less acceptance of premium devices in the industry, leading to the normal growth of the medical imaging equipment industry. The high costs associated with minimally invasive therapies can hinder market growth in developing countries with relatively low income levels.
- Lack of trained individuals
Lack of trained staff and knowledge becomes a challenge, further slowing the pace of market development.
This non-invasive brain trauma monitoring devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the non-invasive brain trauma monitoring devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Non-Invasive Brain Trauma Monitoring Devices Market
The need for remote monitoring and patient engagement solutions has risen dramatically as a result of the COVID-19 outbreak's urgency. In order to provide optimal treatment, most hospitals and healthcare facilities are actively attempting to spread patient monitoring to home care settings or other temporary setups. In contrast to brain monitoring devices, COVID-19 has resulted in a considerable increase in demand for ventilators, and manufacturers are currently focusing their efforts on meeting the growing demand for ventilators, including other breathing devices. The pandemic is expected to have a negative influence on the brain monitoring industry, owing to hospitals' concentration on establishing COVID-19-specific ICUs, as well as the temporary suspension of production and manufacturing facilities in the United States.
Non-Invasive Brain Trauma Monitoring Devices Market Scope
The non-invasive brain trauma monitoring devices market is segmented on the basis of type, product, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Noninvasive Intracranial Pressure Monitor
- Noninvasive Cerebral Edema Dynamic Monitor
- Others
Product
- Consumables
- Electrodes
- Sensors
- Fibre Optic Cables
- Monitoring Devices
- Computerized Tomography (CT) Scanners
- Intracranial Pressure Monitors
- Positron Emission Tomography (PET) Scanners
- Electroencephalogram (EEG)
- Magnetoencephalogram (MEG)
- Others
Application
- Cardiology
- Urology and Nephrology
- Oncology
- Gastroenterology
- Others
End-User
- Hospitals
- Neurological Centers
Non-Invasive Brain Trauma Monitoring Devices Regional Analysis/Insights
The non-invasive brain trauma monitoring devices market is analysed and market size insights and trends are provided by country, type, product, application and end-user as referenced above.
The major countries covered in the non-invasive brain trauma monitoring devices market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
The North American region has emerged as the largest market for non-invasive brain trauma monitoring devices. This is because North America is growing very much with respect to the healthcare industry. North America is expected to dominate the global non-invasive brain trauma monitoring device market geographically due to the presence of multiple technical and pharmaceutical players in countries such as Canada and the United States,
The Asia Pacific region is expected to grow at the highest CAGR during the forecast period of 2022 to 2029 due to higher health care costs and increased awareness of brain-related disorders in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Non-Invasive Brain Trauma Monitoring Devices Market Share Analysis
The non-invasive brain trauma monitoring devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to non-invasive brain trauma monitoring devices market.
Key players operating in the Non-Invasive Brain Trauma Monitoring Devices Market include:
- Advanced Brain Monitoring, Inc.(US)
- Cadwell Industries, Inc.(US)
- Canon Medical Systems Corporation(Japan)
- CAS Medical Systems, Inc.(US)
- Codman & Shurtleff, Inc(US)
- Compumedics Ltd.(Australia)
- Magstim EGI(US)
- General Electric Company(US)
- Hitachi, Ltd.(Japan)
- Koninklijke Philips N.V.(Netherlands)
- Medtronic(US)
- Natus Medical Incorporated(US)
- NeuroLogica Corp.(US)
- NeuroWave Systems, Inc.(US)
- Nihon Kohden Corporation(Japan)
- Noraxon U.S.A., Inc.(US)
- RAUMEDIC AG(Germany)
- Sense Neuro Diagnostics(US)
- Siemens(Germany)
- Sophysa Ltd.(US)
- Spiegelberg GmbH & Co. KG(Germany)
SKU-

