Global Micro Guide Catheters Market
Market Size in USD Million
CAGR :
% 
 USD
188.33 Million
USD
339.14 Million
2024
2032
USD
188.33 Million
USD
339.14 Million
2024
2032
| 2025 –2032 | |
| USD 188.33 Million | |
| USD 339.14 Million | |
|
|
|
|
Micro Guide Catheters Market Size
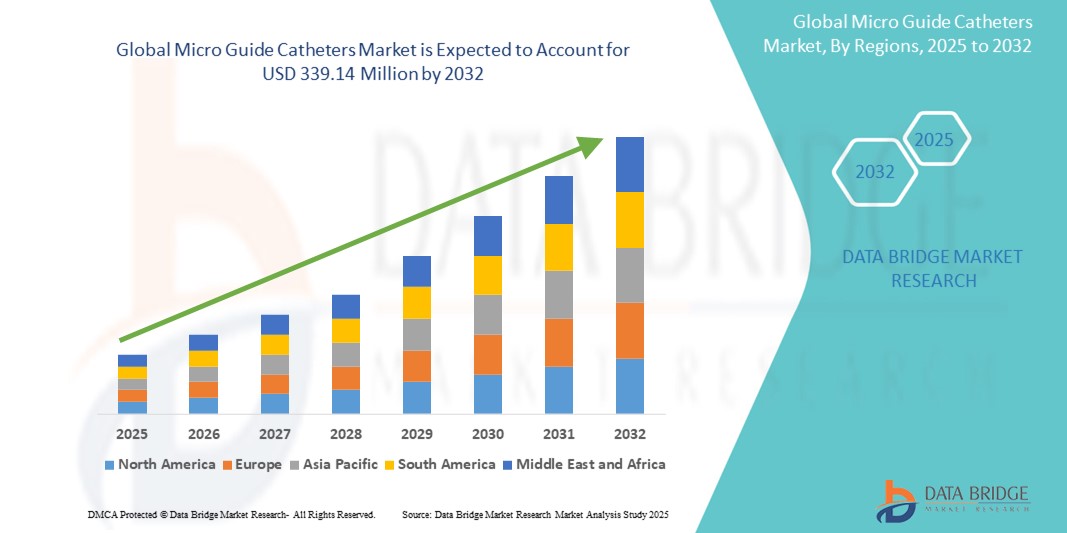
- The global micro guide catheters market size was valued at USD 188.33 million in 2024 and is expected to reach USD 339.14 million by 2032, at a CAGR of 7.63% during the forecast period
- The market growth is largely fueled by the increasing incidence of cardiovascular and neurovascular diseases, which has driven demand for minimally invasive procedures using micro guide catheters for precision navigation within complex vascular pathways
- Furthermore, rising investments in advanced imaging technologies and catheter innovation, along with growing preference for catheter-based interventions over traditional surgeries, are strengthening the adoption of micro guide catheters. These converging factors are accelerating the demand in both developed and emerging healthcare systems, thereby significantly boosting the industry's growth
Micro Guide Catheters Market Analysis
- Micro guide catheters, designed for precise navigation within small and tortuous vasculature, are increasingly vital tools in minimally invasive procedures across neurovascular, cardiovascular, and peripheral vascular interventions due to their flexibility, torque control, and compatibility with advanced imaging systems
- The escalating demand for micro guide catheters is primarily fueled by the rising prevalence of vascular disorders such as stroke, aneurysms, and coronary artery disease, alongside a growing shift toward catheter-based therapeutic procedures over traditional open surgeries
- North America dominated the micro guide catheters market with the largest revenue share of 40.5% in 2024, supported by advanced healthcare infrastructure, early adoption of minimally invasive techniques, and strong presence of leading medical device manufacturers, with the U.S. showing substantial procedural volumes in neurointerventional and cardiac catheterization labs
- Asia-Pacific is expected to be the fastest growing region in the micro guide catheters market during the forecast period due to increasing healthcare expenditure, rapidly expanding hospital infrastructure, and a growing burden of lifestyle-related vascular conditions
- Neurovascular application segment dominated the micro guide catheters market with a market share of 70.5% in 2024, driven by increasing stroke intervention procedures and advancements in catheter technology tailored for complex neurological anatomies
Report Scope and Micro Guide Catheters Market Segmentation
|
Attributes |
Micro Guide Catheters Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Micro Guide Catheters Market Trends
“Miniaturization and Technological Integration Enhancing Precision”
- A significant and accelerating trend in the global micro guide catheters market is the continued miniaturization of catheter devices coupled with integration of advanced imaging and navigation technologies such as 3D road mapping, real-time fluoroscopy, and pressure-sensing capabilities. These innovations are significantly enhancing procedural accuracy and physician control, particularly in complex neurovascular and coronary interventions
- For instance, Penumbra’s latest neurovascular catheters are designed with ultra-low profile tips and flexible shafts for enhanced maneuverability in tortuous vessels, while Philips' image-guided therapy systems are increasingly being used in conjunction with micro guide catheters to improve intraoperative precision
- The integration of micro sensors within catheters allows real-time feedback on vessel wall pressure and catheter positioning, reducing procedural risks and improving patient outcomes. Such advancements are especially critical in treating stroke, aneurysms, and chronic total occlusions, where fine vascular navigation is essential
- Furthermore, manufacturers are focusing on developing hybrid micro catheters that combine steering, tracking, and diagnostic capabilities, enabling a single-device solution for complex interventions
- The growing demand for minimally invasive procedures, especially in aging populations and high-risk cardiovascular patients, is accelerating the adoption of these precision-engineered micro guide catheters across global hospitals and ambulatory centers
- As this trend continues, companies such as Stryker and Medtronic are investing heavily in R&D to produce next-generation devices that deliver superior clinical performance with minimal trauma and maximum compatibility with advanced surgical systems
Micro Guide Catheters Market Dynamics
Driver
“Rising Burden of Vascular Diseases and Preference for Minimally Invasive Procedures”
- The increasing global incidence of vascular disorders, including ischemic stroke, cerebral aneurysms, and coronary artery disease, is a primary driver of growth in the micro guide catheters market. The preference for catheter-based interventions over traditional open surgeries is rising due to shorter recovery times, lower complication risks, and better patient comfort
- For instance, in 2024, Terumo Corporation expanded its portfolio of neurointerventional microcatheters with improved deliverability, targeting hospitals with high stroke caseloads in the U.S. and Europe. These efforts reflect industry-wide momentum toward innovation in interventional solutions
- As healthcare systems globally push for cost-effective and high-efficiency treatments, micro guide catheters offer value by enabling complex procedures in minimally invasive formats. Their utility in both diagnostic and therapeutic roles further adds to their clinical appeal
- The increasing adoption of these catheters in neurovascular, cardiovascular, and peripheral vascular procedures, supported by rising awareness among interventional radiologists and neurologists, continues to strengthen market expansion across developed and emerging markets
Restraint/Challenge
“Complex Manufacturing and Regulatory Barriers”
- Despite the promising growth, the micro guide catheters market faces notable challenges due to the complexity of manufacturing ultra-fine, flexible, and biocompatible catheter designs. Precision engineering demands high-cost raw materials and advanced fabrication technology, contributing to elevated production costs
- Furthermore, strict regulatory approval processes—especially in regions such as the U.S. (FDA) and EU (MDR)—pose hurdles for new product launches. Delays in approvals and clinical validation requirements often extend time-to-market and increase R&D expenditure
- For instance, smaller companies may struggle to meet evolving regulatory standards or obtain sufficient clinical trial data to support approvals, limiting their competitive presence
- In addition, catheter-related complications such as kinking, perforation, or thrombus formation can restrict use in high-risk procedures unless mitigated by improved designs and physician training
- Overcoming these challenges through continued innovation in materials expanded physician education, and faster regulatory pathways will be critical to driving wider market penetration and sustaining long-term growth
Micro Guide Catheters Market Scope
The market is segmented on the basis of type, application, and end user.
- By Type
On the basis of type, the micro guide catheters market is segmented into flow directed micro guide catheters and over-the-wire micro guide catheters. The over-the-wire segment dominated the market with the largest market revenue share of 56.8% in 2024, attributed to its superior navigation capabilities, enhanced support, and compatibility with a wide range of guidewires. These catheters are commonly preferred in complex cardiovascular and peripheral interventions, where enhanced torque and pushability are critical for precise vessel navigation
The flow directed segment is expected to witness the fastest CAGR from 2025 to 2032, driven by increasing adoption in neurovascular interventions, particularly for intracranial aneurysm and AVM (arteriovenous malformation) treatments. These catheters allow for atraumatic navigation through tortuous vessels using blood flow direction, reducing the risk of vessel injury. Their lightweight design and growing utilization in stroke and embolization procedures are contributing to their rising demand.
- By Application
On the basis of application, the micro guide catheters market is segmented into cardiovascular, neurovascular, urology, and other applications. The neurovascular segment led the market with a dominant share of 70.5% in 2024, driven by the increasing prevalence of stroke and the growing preference for minimally invasive neurointerventions. Micro guide catheters play a crucial role in delivering coils, embolic agents, or stents into delicate brain vasculature with high precision. This segment benefits from continuous innovation in microcatheter navigation systems and the expanding neurovascular procedural volume globally.
The Cardiovascular segment is expected to witness the fastest CAGR from 2025 to 2032, due to rising cases of coronary artery disease and peripheral vascular disorders. These catheters are increasingly used in chronic total occlusion (CTO) procedures and percutaneous coronary interventions (PCI), where vessel complexity demands high trackability and support.
- By End User
On the basis of end user, the micro guide catheters market is segmented into hospitals, clinics, and others. The hospital segment held the largest market share in 2024 due to the high volume of catheter-based interventions performed in these settings, supported by advanced infrastructure and availability of specialized medical personnel. Hospitals also tend to adopt new technologies earlier and are often the primary settings for high-complexity procedures involving neuro and cardiovascular catheters.
The clinics segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing establishment of outpatient interventional centers and ambulatory surgical centers (ASCs). These centers are growing in popularity due to their lower procedural costs, reduced patient wait times, and the convenience they offer for minor to moderate catheter-based procedures.
Micro Guide Catheters Market Regional Analysis
- North America dominated the micro guide catheters market with the largest revenue share of 40.5% in 2024, supported by advanced healthcare infrastructure, early adoption of minimally invasive techniques, and strong presence of leading medical device manufacturers
- Healthcare providers in the region prioritize the use of technologically advanced micro catheters due to their precision, flexibility, and ability to navigate complex vascular anatomies, particularly in stroke and coronary interventions
- This dominance is further reinforced by strong R&D investments from key medical device manufacturers, early adoption of innovative catheter technologies, and favorable reimbursement policies, positioning North America as a key hub for both clinical application and innovation in micro guide catheter solutions
U.S. Micro Guide Catheters Market Insight
The U.S. micro guide catheters market captured the largest revenue share of 76.6% within North America in 2024, fueled by a high procedural volume of neurovascular and cardiovascular interventions, early adoption of minimally invasive techniques, and strong presence of key global manufacturers. Advanced healthcare infrastructure and widespread access to cutting-edge interventional tools continue to propel market expansion. In addition, rising incidences of stroke and coronary artery disease, combined with increasing demand for outpatient interventions, reinforce the growing utilization of micro guide catheters in both hospital and ambulatory settings.
Europe Micro Guide Catheters Market Insight
The Europe micro guide catheters market is projected to grow at a substantial CAGR during the forecast period, supported by increasing prevalence of vascular diseases and expanding use of minimally invasive procedures. The region's focus on improving patient outcomes and lowering hospital stays is accelerating the shift toward catheter-based therapies. Moreover, government funding for advanced medical technologies and the implementation of stringent safety standards are encouraging adoption of precision-engineered micro guide catheters across multiple specialties including neurology and cardiology.
U.K. Micro Guide Catheters Market Insight
The U.K. micro guide catheters market is expected to witness notable growth during the forecast period, driven by the rising burden of stroke and peripheral vascular conditions, along with greater investment in interventional radiology. The growing use of digital health technologies and increasing preference for day-case interventions is fostering the deployment of micro guide catheters in NHS and private hospitals asuch as. The U.K.’s strong research infrastructure also supports clinical trials and innovation in catheter design and functionality.
Germany Micro Guide Catheters Market Insight
The Germany micro guide catheters market is poised for steady expansion, supported by a robust healthcare system, high procedural standards, and a strong demand for advanced catheter-based diagnostics and treatments. Germany’s leadership in medical device manufacturing and innovation fosters local availability of precision micro guide catheters. With a high incidence of cardiovascular conditions and an aging population, the need for minimally invasive, safe, and effective therapeutic tools continues to grow.
Asia-Pacific Micro Guide Catheters Market Insight
The Asia-Pacific micro guide catheters market is projected to register the fastest CAGR from 2025 to 2032, driven by rapid healthcare infrastructure development, growing awareness of minimally invasive procedures, and rising disease burden across key economies. Government-led healthcare reforms and investments in tertiary care centers are enabling increased access to interventional technologies, with countries such as China, India, and Japan leading the adoption curve. The region also benefits from a rising pool of trained interventional specialists and growing domestic catheter production capabilities.
Japan Micro Guide Catheters Market Insight
The Japan micro guide catheters market is gaining traction owing to its aging population, strong demand for advanced neurovascular care, and a long-standing emphasis on high-precision medical interventions. Japan is a leader in early adoption of sophisticated catheter-based systems for stroke and aneurysm treatment. Technological innovation, combined with favorable reimbursement policies and government backing for elder healthcare, continues to drive strong market momentum.
India Micro Guide Catheters Market Insight
The India micro guide catheters market is expected to witness rapid growth, driven by the expanding middle class, increasing awareness of minimally invasive treatment options, and government investment in modernizing cardiac and neuro care infrastructure. India is emerging as a major destination for interventional care due to its cost-effective treatment landscape and rising demand from both urban and semi-urban areas. The proliferation of private hospitals and the availability of locally manufactured, affordable micro guide catheters are key factors boosting market expansion.
Micro Guide Catheters Market Share
The micro guide catheters industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Stryker (U.S.)
- Boston Scientific Corporation (U.S.)
- Terumo Corporation (Japan)
- Penumbra, Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Merit Medical Systems (U.S.)
- Asahi Intecc Co., Ltd. (Japan)
- Cook (U.S.)
- Teleflex Incorporated (U.S.)
- Cordis (U.S.)
- Terumo Neuro (U.S.)
- B. Braun SE (Germany)
- Integer Holdings Corporation (U.S.)
- Abbott (U.S.)
- Acandis GmbH (Germany)
- Balton (Poland)
- Reflow Medical, Inc. (U.S.)
- iVascular S.L.U. (Spain)
What are the Recent Developments in Global Micro Guide Catheters Market?
- In April 2024, Medtronic plc announced the global launch of its next-generation micro guide catheter, Echelon 10, designed for enhanced deliverability in neurovascular procedures. This catheter incorporates advanced coil compatibility and improved tracking for treating intracranial aneurysms. The launch represents Medtronic’s continued focus on innovation in minimally invasive neuro-interventional therapies and its effort to expand precision tools for complex cerebrovascular navigation
- In March 2024, Stryker Corporation unveiled its expanded Target Nano Detachable Coil System in Europe and Asia-Pacific markets, optimized for use with high-performance micro guide catheters in neurovascular embolization. The product line is designed to work with ultra-low profile microcatheters, enabling safe treatment of small and tortuous vessels. This expansion enhances Stryker’s neurovascular product ecosystem and reinforces its global market leadership in endovascular solutions
- In February 2024, Terumo Corporation launched a new version of its Progreat Microcatheter System with enhanced pushability and hydrophilic coating for use in complex peripheral interventions. Designed to improve access to distal lesions in lower limb ischemia and AV malformations, the catheter supports physicians performing high-precision therapeutic procedures. This development aligns with Terumo’s strategic focus on vascular health and procedural excellence
- In January 2024, Penumbra, Inc. received FDA clearance for its RED 72 microcatheter, developed specifically for large-vessel aspiration in stroke management. The microcatheter features optimized inner lumen geometry and a flexible distal tip to improve trackability and clot removal efficiency. This regulatory milestone strengthens Penumbra’s neurointerventional portfolio and supports broader access to advanced stroke treatment options
- In December 2023, Boston Scientific Corporation announced the acquisition of Relievant Medsystems, including its catheter technologies used in targeted spinal therapies. While not limited to vascular use, this acquisition reflects a trend toward multi-specialty catheter development and underscores Boston Scientific’s intent to diversify its microcatheter applications across both neurological and structural systems. The integration is expected to stimulate innovation in specialized microcatheter platforms adaptable across therapeutic areas
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.