Global Irritable Bowel Syndrome Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.65 Billion
USD
5.41 Billion
2024
2032
USD
2.65 Billion
USD
5.41 Billion
2024
2032
| 2025 –2032 | |
| USD 2.65 Billion | |
| USD 5.41 Billion | |
|
|
|
|
Irritable Bowel Syndrome Treatment Market Size
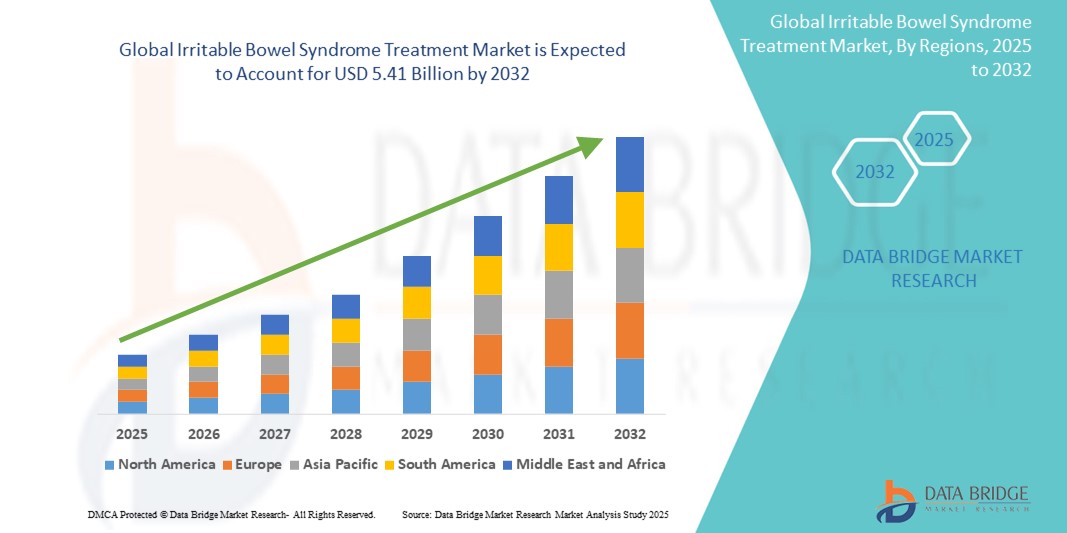
- The global irritable bowel syndrome treatment market size was valued at USD 2.65 billion in 2024 and is expected to reach USD 5.41 billion by 2032, at a CAGR of 9.32% during the forecast period
- The market growth is largely fueled by increasing prevalence of gastrointestinal disorders, coupled with heightened awareness of IBS symptoms and availability of effective therapeutic options, including targeted drugs and dietary solutions
- Furthermore, rising patient demand for personalized and symptom-specific treatment approaches is establishing IBS therapies as a priority area in gastrointestinal care. These converging factors are accelerating research investment and product innovation, thereby significantly boosting the industry’s growth
Irritable Bowel Syndrome Treatment Market Analysis
- Irritable Bowel Syndrome (IBS) treatments, encompassing medications, dietary supplements, and behavioral therapies, are increasingly vital components of gastrointestinal care due to their role in alleviating symptoms such as abdominal pain, bloating, and altered bowel habits in both IBS-C and IBS-D patients
- The escalating demand for IBS treatment is primarily fueled by the growing global prevalence of functional gastrointestinal disorders, rising awareness of IBS-related symptoms, and increasing availability of targeted therapies with fewer side effects
- North America dominated the irritable bowel syndrome treatment market with the largest revenue share of 39.4% in 2024, characterized by high disease awareness, advanced healthcare infrastructure, and the presence of major pharmaceutical players investing in R&D for novel IBS drugs, particularly in the U.S., which sees a high rate of diagnosis and prescription drug uptake
- Asia-Pacific is expected to be the fastest growing region in the irritable bowel syndrome treatment market during the forecast period due to increasing healthcare access, changing dietary habits, and greater diagnosis rates driven by public health initiatives
- The IBS-C segment dominated the irritable bowel syndrome treatment market with a market share of 47% in 2024, driven by the higher patient pool, rising use of prescription treatments such as linaclotide and lubiprostone, and growing clinical research focus on constipation-predominant IBS therapies
Report Scope and Irritable Bowel Syndrome Treatment Market Segmentation
|
Attributes |
Irritable Bowel Syndrome Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Irritable Bowel Syndrome Treatment Market Trends
“Targeted Therapies and Personalized Medicine Approaches”
- A significant and accelerating trend in the global irritable bowel syndrome (IBS) treatment market is the shift towards more targeted, subtype-specific therapies and personalized medicine approaches. This evolution is enhancing treatment efficacy and improving patient outcomes, particularly as the understanding of IBS pathophysiology deepens
- For instance, FDA-approved drugs such as Linzess (linaclotide) for IBS-C and Viberzi (eluxadoline) for IBS-D provide targeted relief based on constipation- or diarrhea-predominant subtypes. Similarly, non-drug interventions such as FODMAP diets and microbiome-focused therapies are gaining popularity among physicians and patients seeking individualized treatment plans
- Advances in diagnostic tools and biomarkers are enabling more accurate classification of IBS subtypes and potential triggers, guiding clinicians in prescribing therapies tailored to the patient’s unique symptom profile. This personalized approach is bolstered by digital health platforms and symptom-tracking apps that help patients and healthcare providers monitor treatment response and make data-driven adjustments
- Pharmaceutical companies are increasingly investing in the development of drugs that address underlying causes rather than just symptom control, including agents that target the gut-brain axis or modulate the microbiome. In addition, new treatment pathways, including serotonin receptor modulators and bile acid modulators, are showing promise in clinical trials
- This trend toward more precise and patient-centric solutions is redefining the IBS treatment paradigm. Consequently, companies such as Ardelyx and Bausch Health are advancing pipeline drugs designed specifically for IBS subpopulations
- The demand for targeted therapies that minimize side effects and deliver consistent, personalized results is growing rapidly across key markets, driven by increased patient awareness, physician advocacy, and regulatory support for innovation in gastrointestinal care
Irritable Bowel Syndrome Treatment Market Dynamics
Driver
“Rising Prevalence and Increasing Awareness of Gastrointestinal Disorders”
- The growing global incidence of gastrointestinal disorders, particularly IBS, combined with rising public and clinical awareness, is a major driver for the increasing demand for IBS treatments
- For instance, according to the International Foundation for Gastrointestinal Disorders, IBS affects an estimated 5–15% of the global population, with many cases going undiagnosed in the past.
- Recent public awareness campaigns and greater emphasis on functional GI disorders in medical education are leading to earlier diagnoses and more targeted treatment
- As more people experience lifestyle-induced digestive issues due to stress, poor diet, and sedentary behavior, healthcare providers are seeing a surge in IBS consultations, prompting both increased pharmaceutical sales and the expansion of therapeutic offerings
- In addition, growing demand for non-invasive, outpatient-based IBS therapies, including dietary interventions and digital therapeutics, reflects broader healthcare trends toward patient-centered care and chronic condition management
Restraint/Challenge
“Misdiagnosis, Symptom Overlap, and High Treatment Costs”
- Despite market growth, challenges such as frequent misdiagnosis and symptom overlap with other gastrointestinal disorders, including inflammatory bowel disease (IBD), continue to hinder optimal treatment outcomes and market penetration
- IBS is a diagnosis of exclusion, often leading to delayed or incorrect treatment. This can erode patient confidence in prescribed therapies and reduce adherence to treatment plans. Furthermore, the heterogeneity of symptoms across IBS subtypes complicates both diagnosis and effective therapy selection
- Another key restraint is the high cost of branded medications and limited insurance coverage in certain regions. While some therapies are available generically, newer treatments such as eluxadoline and linaclotide remain expensive for many patients without robust reimbursement systems
- These factors, along with the psychological burden of a chronic condition with fluctuating symptoms, underscore the need for continued innovation in both diagnostics and affordable, accessible treatment solutions
Irritable Bowel Syndrome Treatment Market Scope
The market is segmented on the basis of type, indication, product, and end use.
- By Type
On the basis of type, the irritable bowel syndrome treatment market is segmented into IBS-C and IBS-D. The IBS-C segment dominated the market with the largest market revenue share of 47% in 2024, driven by a larger patient population and increased adoption of FDA-approved medications such as Linzess (linaclotide) and Amitiza (lubiprostone). These drugs specifically target constipation-predominant symptoms, offering effective symptom relief with well-documented clinical outcomes. Rising awareness among patients and physicians regarding treatment options for IBS-C further boosts this segment’s growth.
The IBS-D segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the availability of advanced treatments such as Viberzi (eluxadoline) and the growing demand for therapies addressing diarrhea-predominant symptoms. Increased diagnostic accuracy and emerging research into gut-brain axis therapies also contribute to the expansion of this segment.
- By Indication
On the basis of indication, the irritable bowel syndrome treatment market is segmented into Irritable Bowel Syndrome with Constipation, Irritable Bowel Syndrome with Diarrhea, and Irritable Bowel Syndrome with Alternating Constipation and Diarrhea. IBS with constipation held the largest share in 2024 due to high global prevalence and increased availability of targeted treatments. The robust sales performance of constipation-focused drugs and favorable reimbursement scenarios in developed countries are primary growth drivers.
The alternating subtype is anticipated to witness the fastest growth rate from 2025 to 2032, as diagnostic challenges give way to improved symptom-tracking tools and tailored treatment protocols. Patients experiencing fluctuating symptoms benefit from flexible treatment strategies, including dietary modulation and behavioral therapy, which are increasingly integrated into care guideline
- By Product
On the basis of product, the irritable bowel syndrome treatment market is segmented into Xifaxan, Linzess/Constella, Viberzi, and Amitiza. Linzess/Constella led the market with the highest revenue share in 2024 due to its broad adoption across North America and Europe for IBS-C treatment and chronic idiopathic constipation. Its proven efficacy, minimal systemic absorption, and favorable safety profile make it a preferred choice for long-term management.
Xifaxan (rifaximin), is anticipated to witness the fastest growth rate from 2025 to 2032, due to increased physician preference for non-systemic antibiotics that modulate gut flora without extensive side effects. Viberzi is gaining market traction for its dual opioid receptor activity, offering improved symptom control in IBS-D cases, while Amitiza continues to be a key option in chronic constipation management among adult women.
- By End Use
On the basis of end use, the irritable bowel syndrome treatment market is segmented into hospitals and clinics and research laboratories. The hospitals and clinics segment held the largest market share in 2024, supported by the high volume of patient visits, specialist care availability, and access to prescription medications. These facilities remain central to diagnosis, treatment planning, and follow-up for IBS, especially in urban settings.
Research laboratories is anticipated to witness the fastest growth rate from 2025 to 2032, due to increased investment in drug discovery, clinical trials, and microbiome research. Pharmaceutical companies and academic institutions are expanding collaborations to better understand IBS etiology and develop next-generation therapeutics, driving innovation and long-term market expansion.
Irritable Bowel Syndrome Treatment Market Regional Analysis
- North America dominated the irritable bowel syndrome treatment market with the largest revenue share of 39.4% in 2024, characterized by high disease awareness, advanced healthcare infrastructure, and the presence of major pharmaceutical players investing in R&D for novel IBS drugs, particularly in the U.S., which sees a high rate of diagnosis and prescription drug uptake
- Patients in the region benefit from early diagnosis, widespread awareness campaigns, and access to FDA-approved targeted therapies such as Linzess, Viberzi, and Xifaxan, contributing to robust treatment adoption across both primary care and specialist settings
- This widespread uptake is further supported by well-established healthcare infrastructure, proactive physician engagement, and strong pharmaceutical company presence, positioning North America as a key region in shaping future innovations and adoption trends in IBS management
U.S. Irritable Bowel Syndrome Treatment Market Insight
The U.S. irritable bowel syndrome treatment market captured the largest revenue share in North America in 2024, fueled by the country’s high prevalence of functional gastrointestinal disorders and the availability of advanced therapies. Growing awareness about IBS symptoms, combined with strong physician engagement and patient education efforts, is driving early diagnosis and treatment uptake. In addition, favorable reimbursement policies and active research into novel therapeutics are supporting market expansion. The presence of major pharmaceutical players with strong IBS-focused portfolios further enhances the country’s market leadership.
Europe Irritable Bowel Syndrome Treatment Market Insight
The Europe irritable bowel syndrome treatment market is projected to expand at a steady CAGR throughout the forecast period, driven by increasing diagnosis rates and growing awareness of gastrointestinal health. The rising demand for effective and tolerable treatment options is fostering the uptake of newer drug classes and dietary interventions. With national health systems supporting early-stage diagnosis and prescription coverage, the region is witnessing growing demand across general practitioners and specialist clinics. The integration of personalized treatment strategies and the adoption of microbiome-focused therapies are expected to support long-term growth.
U.K. Irritable Bowel Syndrome Treatment Market Insight
The U.K. irritable bowel syndrome treatment market is anticipated to grow at a notable CAGR during the forecast period, driven by strong public health infrastructure and rising awareness campaigns about digestive disorders. Increasing attention to gut health and mental well-being, supported by NHS-backed initiatives, is encouraging more patients to seek diagnosis and care. Digital health tools and symptom management apps are also enhancing patient engagement and adherence to treatment regimens. These factors, alongside expanded availability of IBS-specific medications, are expected to accelerate market growth.
Germany Irritable Bowel Syndrome Treatment Market Insight
The Germany irritable bowel syndrome treatment market is expected to grow at a significant CAGR during the forecast period, fueled by the country's emphasis on medical innovation and preventive healthcare. German consumers show strong interest in digestive health, with rising adoption of both pharmaceutical and dietary-based IBS therapies. Clinical trials and academic research institutions in Germany are also contributing to drug development and personalized care approaches. The country's universal healthcare system ensures wide accessibility to IBS treatment, making it a key contributor to the European market.
Asia-Pacific Irritable Bowel Syndrome Treatment Market Insight
The Asia-Pacific irritable bowel syndrome treatment market is poised to grow at the fastest CAGR of 10.3% during the forecast period of 2025 to 2032, driven by increasing awareness, urbanization, and lifestyle changes in countries such as China, Japan, and India. Rising incidence of digestive disorders and stress-related gastrointestinal symptoms are contributing to higher demand for IBS therapies. Government efforts to strengthen healthcare infrastructure and improve access to specialty care are also fueling regional growth. In addition, the expansion of pharmaceutical manufacturing and clinical trials in APAC supports affordability and market penetration.
Japan Irritable Bowel Syndrome Treatment Market Insight
The Japan irritable bowel syndrome treatment market is gaining momentum due to a high prevalence of gastrointestinal sensitivity and the population’s growing focus on overall wellness. The integration of traditional and modern therapeutic approaches is common in Japan, with patients seeking symptom relief through both prescription medications and dietary strategies. Technological advancements in diagnostics and personalized care tools are further supporting treatment optimization. Japan’s aging population is also driving demand for safe, tolerable long-term IBS solutions.
India Irritable Bowel Syndrome Treatment Market Insight
The India irritable bowel syndrome treatment market accounted for the largest market revenue share in Asia Pacific in 2024, driven by increased diagnosis rates, changing diets, and high healthcare consumer engagement. Urbanization, stress, and dietary shifts are leading to a surge in IBS cases, particularly among the working-age population. The availability of cost-effective therapies and increased patient access to gastroenterologists in metropolitan areas are boosting treatment rates. Local pharmaceutical manufacturers and growing awareness of gut health through digital platforms are expected to sustain strong market momentum.
Irritable Bowel Syndrome Treatment Market Share
The irritable bowel syndrome treatment industry is primarily led by well-established companies, including:
- Ironwood (U.S.)
- AbbVie Inc. (U.S.)
- Astellas Pharma, Inc. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
- AstraZeneca (U.K.)
- Sebela Pharmaceuticals (U.S.)
- Bausch Health Companies Inc (Canada)
- Theriva Biologics (U.S.)
- Ardelyx (U.S.)
- Salix Pharmaceuticals (U.S.)
- Mallinckrodt (U.S.)
- Abbott (U.S.)
- Lexicon Pharmaceuticals, Inc. (U.S.)
- GSK plc (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Ono Pharmaceutical Co., Ltd. (Japan)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
What are the Recent Developments in Global Irritable Bowel Syndrome Treatment Market?
- In May 2024, Ardelyx, Inc. announced the positive Phase 3 trial results of its investigational therapy tenapanor for IBS-C, showcasing significant improvements in abdominal pain and stool consistency. This milestone reinforces the company’s commitment to delivering innovative, targeted treatments and marks a potential expansion in therapeutic options for constipation-predominant IBS patients. Ardelyx’s focus on sodium/hydrogen exchanger inhibitors represents a novel approach in gastrointestinal pharmacology, contributing to the evolving landscape of IBS care
- In March 2024, Bausch Health Companies Inc. and its gastroenterology arm Salix Pharmaceuticals expanded access to Xifaxan (rifaximin) in Europe through strategic partnerships. This move is aimed at strengthening the company’s international presence and improving treatment availability for IBS-D patients. The collaboration enhances distribution efficiency and supports physician awareness campaigns, signaling the firm’s intent to extend its global reach in the IBS therapeutics market
- In February 2024, Ironwood Pharmaceuticals, Inc. entered into a collaboration with a digital health platform to develop AI-powered tools for IBS-C symptom tracking and personalized treatment support. This initiative reflects the growing integration of digital technology into chronic gastrointestinal disorder management, empowering patients with real-time monitoring and data-driven therapeutic recommendations. Ironwood aims to improve medication adherence and optimize outcomes for patients using Linzess (linaclotide)
- In January 2024, the U.S. FDA granted Fast Track designation to 9 Meters Biopharma’s NM-002, a long-acting injectable GLP-1 receptor agonist for the treatment of IBS-D. This designation accelerates the regulatory review process and highlights the potential of NM-002 as a first-in-class therapy addressing key unmet needs in the diarrhea-predominant IBS population. The development marks a significant step forward in diversifying treatment mechanisms and improving patient outcomes
- In December 2023, AlfaSigma S.p.A. launched a new formulation of rifaximin in select Asia-Pacific markets aimed at improving compliance and symptom relief in IBS-D. With a strong emphasis on microbiota modulation and non-systemic action, the updated formulation seeks to enhance tolerability and efficacy. This development demonstrates AlfaSigma’s commitment to expanding access to evidence-based IBS treatments, particularly in emerging markets where awareness and diagnosis rates are increasing
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.