Global Idiopathic Pulmonary Fibrosis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.12 Billion
USD
6.08 Billion
2024
2032
USD
3.12 Billion
USD
6.08 Billion
2024
2032
| 2025 –2032 | |
| USD 3.12 Billion | |
| USD 6.08 Billion | |
|
|
|
|
Idiopathic Pulmonary Fibrosis Treatment Market Size
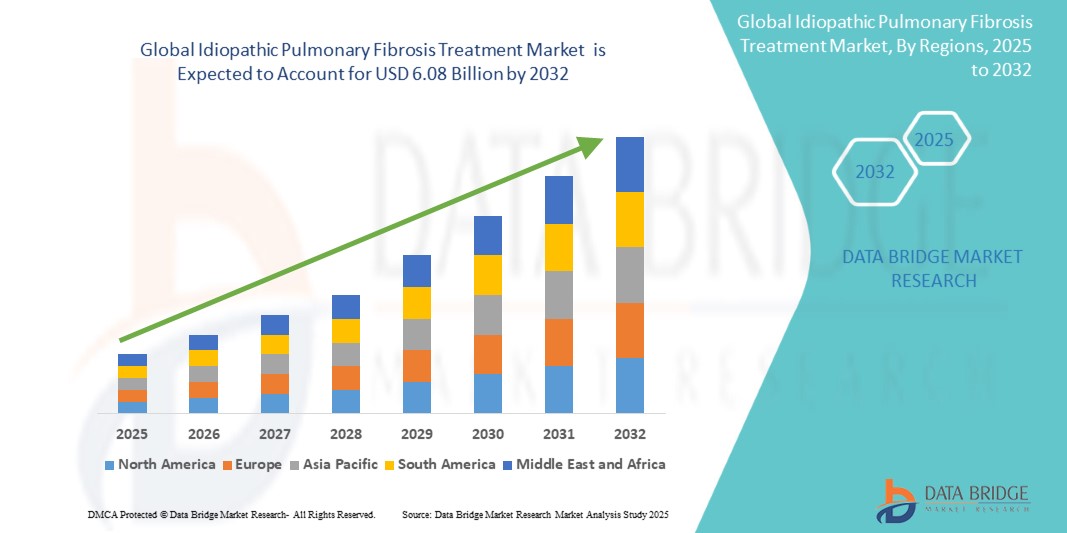
- The Global Idiopathic Pulmonary Fibrosis Treatment Market size was valued at USD 3.12 billion in 2024 and is expected to reach USD 6.08 billion by 2032, at a CAGR of 9.2% during the forecast period
- This growth is driven by factors such as the rising incidence of idiopathic pulmonary fibrosis, an aging global population, and ongoing advancements in diagnostic and therapeutic technologies
Idiopathic Pulmonary Fibrosis Treatment Market Analysis
- Idiopathic pulmonary fibrosis is a chronic, progressive fibrotic interstitial lung disease of unknown cause, characterized by worsening dyspnea and lung function. The increasing incidence of IPF, advancements in diagnostic tools, and growing awareness among physicians and patients are key factors driving the treatment market
- The demand for these microscopes is significantly driven by their disease-modifying potential and regulatory approvals in multiple regions
- North America holds the largest share in the global IPF treatment market, owing to the presence of major pharmaceutical players, favorable reimbursement policies, and strong R&D pipeline
- Asia-Pacific is anticipated to register the highest CAGR due to a growing aging population, increasing diagnostic rates, and improving healthcare infrastructure
- Tyrosine Inhibitors are expected to dominate the market in 2025 with the largest market share of 47.2%, driven by clinical efficacy in slowing disease progression and regulatory support
Report Scope and Idiopathic Pulmonary Fibrosis Treatment Market Segmentation
|
Attributes |
Idiopathic Pulmonary Fibrosis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Idiopathic Pulmonary Fibrosis Treatment Market Trends
“Rise of Personalized Biologic Therapies and Digital Health Integration”
- The IPF treatment landscape is witnessing a significant transformation with the growing adoption of personalized medicine. Biologic therapies, such as nintedanib (an antifibrotic agent approved for IPF and progressive fibrosing interstitial lung diseases) and tocilizumab (originally used in rheumatoid arthritis and being explored for fibrotic lung diseases), are increasingly tailored based on genetic profiling and patient-specific biomarkers
- Advances in genomic sequencing and transcriptomic analysis are enabling clinicians to identify unique disease subtypes and progression patterns, making it possible to deliver more targeted and effective treatments.
- For Instances, In 2021, nintedanib (Ofev, by Boehringer Ingelheim) was included in multiple studies investigating biomarker-based patient stratification, including the INBUILD trial, which examined its effects across various fibrosing interstitial lung diseases, including IPF.
- Moreover, the integration of digital health technologies, such as wearable devices and remote e-monitoring platforms, is revolutionizing disease management. Devices like Bluetooth-enabled spirometers allow real-time monitoring of lung function, while mobile applications can track medication adherence and symptom progression
Idiopathic Pulmonary Fibrosis Treatment Market Dynamics
Driver
“High Prevalence of IPF and Favorable Regulatory Landscape”
- Idiopathic Pulmonary Fibrosis affects over 5 million individuals globally, and its prevalence continues to rise, particularly among populations aged 60 years and above. The disease’s etiology remains unclear, and it is associated with rapid lung function decline, significantly impairing quality of life and survival.
- This increasing disease burden is pushing governments and regulatory agencies to prioritize IPF therapies. Agencies such as the U.S. FDA, European Medicines Agency (EMA), and PMDA in Japan have granted orphan drug designations and accelerated approval pathways to key treatments like pirfenidone (Esbriet) and nintedanib (Ofev).
- These regulatory incentives lower development costs, shorten time-to-market, and encourage pharmaceutical companies to invest in IPF research.
For instance,
- A 2022 report by the Global Burden of Disease (GBD) estimated over 5 million global IPF cases, with highest incidence in populations aged 60 and older
- As a result of the rising prevalence of idiopathic pulmonary fibrosis—particularly among the aging population there is a significant increase in demand for effective IPF treatments, supported by a favorable regulatory landscape that accelerates the approval and availability of novel antifibrotic therapies
Opportunity
“Emerging Role of Combination Therapies and Companion Diagnostics”
- To overcome the limitations of monotherapy, researchers are exploring combination regimens that integrate antifibrotics, anti-inflammatory agents, and biologics.
- These multidimensional approaches aim to target multiple pathological pathways involved in IPF—such as fibrosis, inflammation, and immune dysregulation.
- At the same time, the use of companion diagnostics is becoming crucial in stratifying patients who are more likely to respond to specific therapies. Tools like gene expression assays, developed by companies like Veracyte, help identify molecular subtypes of IPF and predict treatment responses.
For instance,
- In 2022, the INJOURNEY trial (sponsored by Boehringer Ingelheim) investigated the safety and efficacy of combining nintedanib with pirfenidone, showing tolerability and potential for additive effects in reducing lung function decline
- The integration of companion diagnostics and AI-driven biomarker analysis in IPF care is enabling more precise patient stratification, leading to improved treatment outcomes and optimized therapeutic decisions..
Restraint/Challenge
“High Cost of Treatment and Limited Curative Options”
- Despite the availability of antifibrotic therapies, the high cost of IPF treatment remains a major barrier to access.
- Annual treatment costs for drugs like nintedanib and pirfenidone can exceed $100,000 per patient, placing a heavy burden on healthcare systems and patients, especially in low- and middle-income countries.
- This financial barrier often leads to delayed initiation or premature discontinuation of therapy, negatively impacting patient outcomes.
For instance,
- As of 2024, annual treatment costs for nintedanib and pirfenidone remain between $90,000 and USD 110,000 per patient in the U.S., according to data from GoodRx and the National Institute for Health and Care Excellence (NICE)
- Consequently, the high cost of antifibrotic therapies and the limited availability of curative options such as lung transplantation can lead to significant disparities in access to treatment, particularly in low- and middle-income regions, thereby hindering the overall growth and equity of the global IPF treatment market.
Idiopathic Pulmonary Fibrosis Treatment Market Scope
The market is segmented on the basis application, product type, technology, magnification type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Class |
|
|
By Marketed Drug |
|
|
By Medication Type |
|
|
By Type
|
|
|
By Route of Administration |
|
|
By End User
|
|
|
By Distribution Channel |
|
In 2025, the Tyrosine Inhibitors is projected to dominate the market with a largest share in drug Class segment
In 2025, the antifibrotic agents segment is projected to dominate with a 47.2% market share due to regulatory approvals, disease-modifying effects, and wide prescription rates of drugs like pirfenidone and nintedanib. The oral route is expected to account for the largest share during the forecast period
The Ofev is expected to account for the largest share during the forecast period in technology market
In 2025, the oral route of administration is anticipated to hold the largest market share of 68.4% due to convenience, better patient adherence, and availability of key antifibrotic therapies in oral form IPF Treatment Market Regional Analysis.
Idiopathic Pulmonary Fibrosis Treatment Market Regional Analysis
“North America Holds the Largest Share in the Idiopathic Pulmonary Fibrosis Treatment Market”
- North America leads the global IPF treatment market due to well-established reimbursement systems, high awareness, and the presence of leading pharmaceutical companies
- The U.S. remains the primary contributor due to high diagnosis rates, access to novel drugs, and strong clinical trial participation
- The availability of well-established reimbursement policies and growing investments in research & development by leading medical device companies further strengthen the market.
- In addition, the increasing number of diagnosed IPF cases, along with growing adoption of advanced therapeutic approaches such as antifibrotic agents and minimally invasive diagnostic techniques, is fueling market expansion across key regions
“Asia-Pacific is Projected to Register the Highest CAGR in the Idiopathic Pulmonary Fibrosis Treatment Market”
- Asia-Pacific is expected to grow at the highest CAGR, fueled by growing elderly population, rising disease recognition, and improving healthcare access
- Countries such as China, India, and Japan are investing in IPF screening programs and early intervention strategies
- Japan, with its advanced healthcare infrastructure and strong focus on respiratory disease research, remains a crucial market for IPF treatment. The country continues to lead in the adoption of novel antifibrotic therapies and precision diagnostic tools, supported by favorable regulatory frameworks and high healthcare spending
- China and India, with their large aging populations and increasing incidence of idiopathic pulmonary fibrosis, are witnessing significant growth in public and private investments in pulmonary care. The expanding presence of global biopharmaceutical companies, rising awareness about IPF, and improved access to specialized diagnostics and treatment options are driving substantial market growth across both countries
Idiopathic Pulmonary Fibrosis Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Boehringer Ingelheim International GmbH (Germany)
- GNI Group Ltd. (Japan)
- MediciNova, Inc. (U.S.)
- Cipla Inc. (India)
- Bristol-Myers Squibb Company (U.S.)
- Galapagos NV (Belgium
- United Therapeutics Corporation (U.S.)
- FibroGen, Inc. (U.S.)
- Veracyte, Inc. (U.S.)
Latest Developments in Global Idiopathic Pulmonary Fibrosis Treatment Market
- In September 2024, Boehringer Ingelheim announced that its FIBRONEER-IPF study of nerandomilast successfully met its primary endpoint, prompting plans for a new drug application for IPF treatment.
- In May 2024, Ferrer expanded its distribution agreement with United Therapeutics to obtain worldwide rights for treprostinil inhalation solution, targeting potential indications for Idiopathic and Progressive Pulmonary Fibrosis
- In September 2024, Boehringer Ingelheim announced that its FIBRONEER-IPF study of nerandomilast successfully met its primary endpoint, prompting plans for a new drug application for IPF treatment.
- In May 2024, Ferrer expanded its distribution agreement with United Therapeutics to obtain worldwide rights for treprostinil inhalation solution, targeting potential indications for Idiopathic and Progressive Pulmonary Fibrosis
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.