Global Ethical Pharmaceuticals Market
Market Size in USD Billion
CAGR :
% 
 USD
5.38 Billion
USD
10.12 Billion
2024
2032
USD
5.38 Billion
USD
10.12 Billion
2024
2032
| 2025 –2032 | |
| USD 5.38 Billion | |
| USD 10.12 Billion | |
|
|
|
|
Ethical Pharmaceuticals Market Size
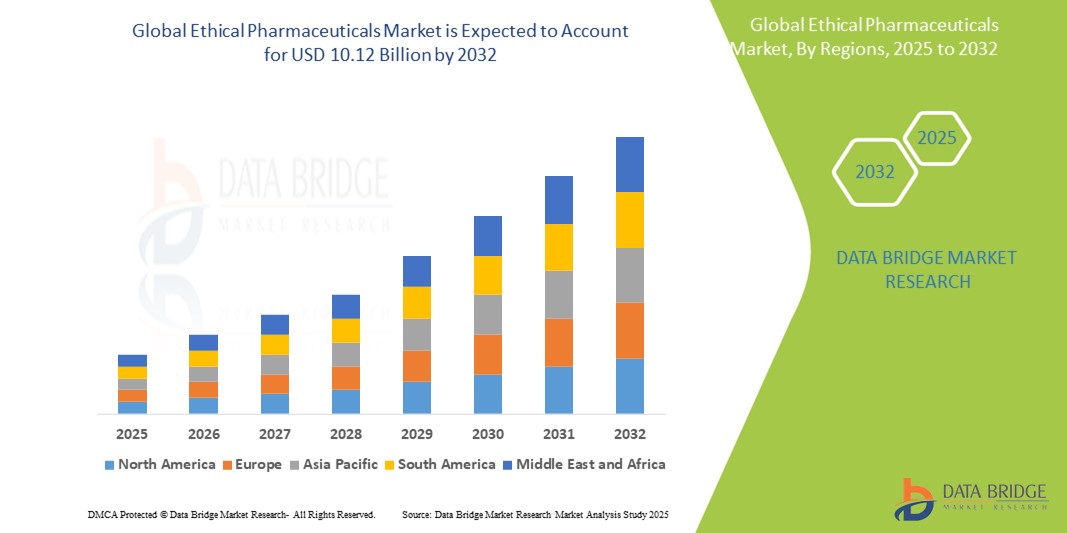
- The global ethical pharmaceuticals market size was valued at USD 5.38 billion in 2024 and is expected to reach USD 10.12 billion by 2032, at a CAGR of 8.20% during the forecast period
- The market growth is largely driven by increasing prevalence of chronic diseases, aging populations, and rising healthcare expenditure, especially in emerging economies, which is propelling demand for prescription-based medication
- Furthermore, expanding access to healthcare services, advancements in drug development technologies, and robust pipelines across therapeutic areas are reinforcing the critical role of ethical pharmaceuticals in modern medicine. These combined factors are contributing significantly to the market’s upward trajectory, positioning it as a cornerstone of the global pharmaceutical landscape
Ethical Pharmaceuticals Market Analysis
- Ethical pharmaceuticals, referring to prescription drugs dispensed only through authorized medical channels, play a pivotal role in modern healthcare systems due to their regulated distribution, therapeutic efficacy, and physician-led administration across diverse medical conditions
- The rising demand for ethical pharmaceuticals is primarily driven by the global surge in chronic diseases, increased healthcare access in developing regions, and an aging global population requiring long-term treatments for complex conditions
- North America dominated the ethical pharmaceuticals market with the largest revenue share of 42.8% in 2024, attributed to strong healthcare infrastructure, high R&D spending, favorable reimbursement policies, and the presence of leading pharmaceutical companies with robust drug pipelines and innovative therapies
- Asia-Pacific is expected to be the fastest growing region in the ethical pharmaceuticals market during the forecast period due to improving healthcare infrastructure, expanding insurance coverage, and increasing investment in local drug manufacturing and regulatory harmonization
- Hospitals and clinics segment dominated the ethical pharmaceuticals market with a market share of 49.1% in 2024, driven by its increasing number of hospital admissions, chronic disease treatments, and institutional reliance on prescription-based pharmaceuticals administered under medical supervision
Report Scope and Ethical Pharmaceuticals Market Segmentation
|
Attributes |
Ethical Pharmaceuticals Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ethical Pharmaceuticals Market Trends
“Rise of Personalized Medicine and Biologic Therapies”
- A key and rapidly growing trend in the global ethical pharmaceuticals market is the increasing focus on personalized medicine and the development of biologics, which are revolutionizing treatment approaches for complex diseases such as cancer, autoimmune disorders, and rare genetic conditions
- For instance, monoclonal antibodies such as Keytruda (Merck) and Humira (AbbVie) have become mainstays in treating various cancers and inflammatory diseases, offering highly targeted and effective therapies with fewer side effects. Similarly, CAR-T cell therapies are being developed for specific patient profiles in hematologic cancers
- Personalized medicine leverages genomic information and biomarkers to tailor treatments based on individual genetic profiles, enhancing therapeutic outcomes and minimizing trial-and-error prescriptions. Increasing collaborations between pharmaceutical companies and genomics firms are accelerating this shift
- Biologics and biosimilars, which are more complex and specific than traditional small-molecule drugs, are gaining traction due to their efficacy in managing previously difficult-to-treat conditions. For instance, Regeneron and Roche are actively investing in large-scale biologic pipelines across oncology and immunology
- The trend is also reflected in regulatory support, as authorities such as the FDA and EMA expedite approval pathways for breakthrough and orphan drugs. This regulatory momentum is encouraging pharmaceutical players to prioritize innovation in biologics and personalized therapeutics
- As patient expectations evolve, the demand for more effective, individualized treatments is intensifying, prompting major ethical pharmaceutical companies to invest in advanced R&D capabilities, precision drug delivery, and companion diagnostics, thereby reshaping the competitive landscape and future growth trajectory
Ethical Pharmaceuticals Market Dynamics
Driver
“Increasing Chronic Disease Burden and Aging Global Population”
- The rising prevalence of chronic illnesses such as diabetes, cardiovascular disorders, respiratory conditions, and cancer, coupled with the globally aging population, is a significant driver propelling the demand for ethical pharmaceuticals
- For instance, according to the WHO, noncommunicable diseases account for nearly 74% of all global deaths. This surge in long-term disease management requires consistent access to regulated prescription medicines, solidifying the role of ethical drugs in global healthcare systems
- The elderly population, particularly in high- and middle-income countries, is growing rapidly, contributing to higher prescription drug utilization and driving pharmaceutical companies to expand therapeutic options for age-related ailments such as Alzheimer’s, arthritis, and osteoporosis
- Further, the expansion of universal healthcare programs in emerging markets is improving access to prescription medications, reinforcing demand across hospital and retail channels. These developments are complemented by growing insurance coverage and improved diagnostic capabilities, which are fueling timely interventions and ongoing pharmacological treatment
- Multinational pharmaceutical companies are responding to these drivers by increasing investments in R&D, launching patient support initiatives, and forming alliances with healthcare providers to ensure continuity of care. These strategies collectively accelerate market penetration and long-term growth potential for ethical drugs
Restraint/Challenge
“Patent Expiry and Regulatory Compliance Challenges”
- A major restraint facing the ethical pharmaceuticals market is the expiration of patents on blockbuster drugs, leading to revenue erosion and heightened competition from generic alternatives and biosimilars. For instance, the loss of exclusivity for drugs such as Humira in key markets has opened the door for lower-cost competitors, impacting the profit margins of originator companies
- In addition, the highly regulated nature of the ethical pharmaceuticals industry imposes significant barriers in terms of compliance, approval timelines, and post-market surveillance. Regulatory frameworks across geographies such as the FDA (U.S.), EMA (Europe), and CDSCO (India) often differ, requiring companies to navigate complex and costly approval processes
- The stringent clinical trial requirements, coupled with increasing expectations for real-world evidence and pharmacovigilance, extend product development timelines and inflate operational costs. This can especially impact small and mid-sized pharmaceutical firms that lack robust regulatory infrastructure
- Moreover, frequent changes in drug pricing regulations, reimbursement policies, and public scrutiny over pharmaceutical costs have created uncertainty in long-term planning. These dynamics demand that companies remain agile and transparent in their pricing strategies while maintaining compliance
- Addressing these challenges will require continuous innovation, investment in regulatory expertise, collaborative partnerships with health authorities, and lifecycle management strategies including drug reformulations and combination therapies to extend product viability and competitiveness.
Ethical Pharmaceuticals Market Scope
The market is segmented on the basis of therapeutic class and application.
- By Therapeutic Class
On the basis of therapeutic class, the ethical pharmaceuticals market is segmented into lipid regulators, narcotic analgesics, ACE inhibitors, respiratory agents, diuretics, calcium antagonists, hormonal contraceptives, penicillin, vitamins, and minerals. The lipid regulators segment dominated the market with the largest market revenue share in 2024, driven by the rising incidence of cardiovascular diseases and the increasing use of statins as a standard therapy for lowering LDL cholesterol. These drugs are widely prescribed across developed and emerging markets due to their strong clinical efficacy and insurance coverage. The long-term benefits of lipid regulators in preventing strokes and heart attacks further support segment growth.
The respiratory agents segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing global cases of asthma, COPD, and respiratory infections. The post-pandemic focus on respiratory health, coupled with advances in inhalation drug delivery technologies and growing adoption of combination therapies, is driving rapid expansion. Rising pollution levels and smoking prevalence in developing countries are also contributing to heightened demand for respiratory medications.
- By Application
On the basis of application, the ethical pharmaceuticals market is segmented into hospitals and clinics, pharmaceutical companies, and others. The hospitals and clinics segment held the largest market revenue share of 49.1% in 2024, driven by the high volume of inpatient and outpatient treatments requiring ethical prescription drugs. The presence of skilled professionals, extensive formularies, and access to critical care infrastructure support the dominance of this segment. Public and private hospitals also benefit from government-subsidized medicine supply chains and structured procurement practices.
The pharmaceutical companies segment is expected to witness the fastest CAGR from 2025 to 2032, driven by increasing R&D investment, drug formulation advancements, and rising demand for clinical trial supplies. Companies rely on ethical pharmaceuticals during each stage of drug development, from pre-clinical to post-marketing trials, making them a core operational need. Collaboration with research organizations and expansion in biosimilar and specialty drug manufacturing are also driving segment growth.
Ethical Pharmaceuticals Market Regional Analysis
- North America dominated the ethical pharmaceuticals market with the largest revenue share of 42.8% in 2024, attributed to strong healthcare infrastructure, high R&D spending, favorable reimbursement policies, and the presence of leading pharmaceutical companies with robust drug pipelines and innovative therapies
- The region's demand is further fueled by advanced R&D capabilities, a rising elderly population, and broad insurance coverage that facilitates access to ethical drugs across demographics
- This sustained growth is also supported by high healthcare spending, the presence of major pharmaceutical companies, and continuous drug innovation, making North America a key hub for ethical pharmaceutical consumption and development
U.S. Ethical Pharmaceuticals Market Insight
The U.S. ethical pharmaceuticals market captured the largest revenue share of 79.3% in 2024 within North America, driven by strong healthcare infrastructure, an aging population, and growing demand for prescription-based therapies. The nation’s regulatory support for branded medications, along with rapid advancements in drug development and personalized medicine, further propel growth. High rates of chronic diseases, including diabetes and cardiovascular disorders, along with significant investment in R&D by major pharmaceutical companies, continue to reinforce the market’s dominance.
Europe Ethical Pharmaceuticals Market Insight
The Europe ethical pharmaceuticals market is projected to register robust growth during the forecast period, fueled by increased healthcare funding and stringent drug approval standards that support high-quality treatment options. Rising incidence of age-related ailments, growing awareness of evidence-based therapies, and government-backed reimbursement policies are encouraging the uptake of ethical drugs. The region also benefits from a strong focus on innovation and cross-border collaboration among pharmaceutical companies.
U.K. Ethical Pharmaceuticals Market Insight
The U.K. ethical pharmaceuticals market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by a mature healthcare system and a strong pipeline of clinical research. Government initiatives aimed at strengthening public health infrastructure and addressing unmet medical needs drive ethical drug adoption. The NHS’s emphasis on high-standard prescription practices and increased investment in life sciences research contribute to market expansion, with oncology, diabetes, and cardiovascular segments showing notable growth.
Germany Ethical Pharmaceuticals Market Insight
The Germany ethical pharmaceuticals market is expected to experience steady growth during the forecast period, backed by its advanced medical sector and high pharmaceutical R&D expenditure. Strict regulatory controls and a strong preference for branded medications ensure wide-scale adoption of ethical drugs. Germany’s robust insurance coverage and its status as a leading pharmaceutical manufacturing base in Europe make it a vital market for prescription therapies, especially in oncology, immunology, and cardiovascular treatment.
Asia-Pacific Ethical Pharmaceuticals Market Insight
The Asia-Pacific ethical pharmaceuticals market is set to grow at the fastest CAGR from 2025 to 2032, driven by rising healthcare access, growing population, and increased incidence of chronic diseases. Countries such as China, Japan, and India are investing heavily in public health and digital health infrastructure, facilitating greater penetration of ethical drugs. Moreover, the expansion of multinational pharmaceutical firms into the region and supportive government policies are key drivers of rapid growth.
Japan Ethical Pharmaceuticals Market Insight
The Japan ethical pharmaceuticals market is growing steadily due to its aging demographic and national focus on healthcare quality. Government policies supporting innovation, such as accelerated drug approval programs and robust reimbursement models, are aiding market expansion. Japan’s long-standing leadership in biotechnology and pharmaceuticals, combined with patient-centric care models, reinforces ethical drug adoption, particularly in oncology, neurology, and rare disease segments.
India Ethical Pharmaceuticals Market Insight
The India ethical pharmaceuticals market held the largest revenue share in Asia Pacific in 2024, driven by expanding healthcare coverage, rising middle-class demand for prescription drugs, and increased awareness of chronic illness management. The market benefits from domestic manufacturing strength and growing investments in research and regulatory compliance. India's push toward universal health access and affordable branded therapies is fostering widespread adoption of ethical pharmaceuticals across both urban and rural settings.
Ethical Pharmaceuticals Market Share
The ethical pharmaceuticals industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- Merck & Co., Inc. (U.S.)
- Sanofi (France)
- AstraZeneca (U.K.)
- GSK plc (U.K.)
- AbbVie Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Bayer AG (Germany)
- Amgen Inc. (U.S.)
- Lilly (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Boehringer Ingelheim International GmbH (Germany)
- Novo Nordisk A/S (Denmark)
- Astellas Pharma Inc. (Japan)
- Otsuka Pharmaceutical Co., Ltd. (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sun Pharmaceutical Industries Ltd. (India)
What are the Recent Developments in Global Ethical Pharmaceuticals Market?
- In April 2023, Pfizer Inc. announced the expansion of its oncology pipeline through a strategic partnership with Arvinas, Inc. The collaboration focuses on the co-development of novel protein degrader therapies targeting difficult-to-treat cancers. This initiative emphasizes Pfizer’s commitment to advancing ethical pharmaceuticals by investing in innovative, precision-based treatment solutions to address unmet medical needs in oncology
- In March 2023, AstraZeneca Plc launched its Sustainable Markets Initiative Health Systems Task Force pilot program across several low- and middle-income countries. The program aims to improve access to ethical medicines through more efficient supply chain models and public-private collaborations. The initiative underlines AstraZeneca's dedication to equitable healthcare and its leadership role in delivering high-standard prescription treatments globally
- In February 2023, Johnson & Johnson's Janssen Pharmaceuticals received FDA approval for its new ethical prescription therapy, Tepmetko, indicated for patients with metastatic non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping alterations. This approval marks a significant milestone in personalized oncology treatments, reinforcing the company’s role in delivering clinically validated, targeted therapies under strict regulatory compliance
- In February 2023, Novartis AG partnered with the African Medical Supplies Platform (AMSP) to enhance the availability of branded cardiovascular and diabetes medications across sub-Saharan Africa. The agreement supports Novartis' ethical drug distribution initiatives and aims to reduce disease burden in underserved regions through affordable access to essential, high-quality medications
- In January 2023, Merck & Co., Inc. launched a global access program for its immunotherapy drug Keytruda, aiming to increase affordability and accessibility in low-income countries. This development reflects Merck’s ongoing commitment to expanding global access to cutting-edge ethical pharmaceuticals, particularly in oncology, while maintaining the highest standards of safety, efficacy, and patient-centric innovation
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.