Global Endotoxin And Pyrogen Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
1.27 Billion
USD
2.98 Billion
2024
2032
USD
1.27 Billion
USD
2.98 Billion
2024
2032
| 2025 –2032 | |
| USD 1.27 Billion | |
| USD 2.98 Billion | |
|
|
|
|
Endotoxin and Pyrogen Testing Market Size
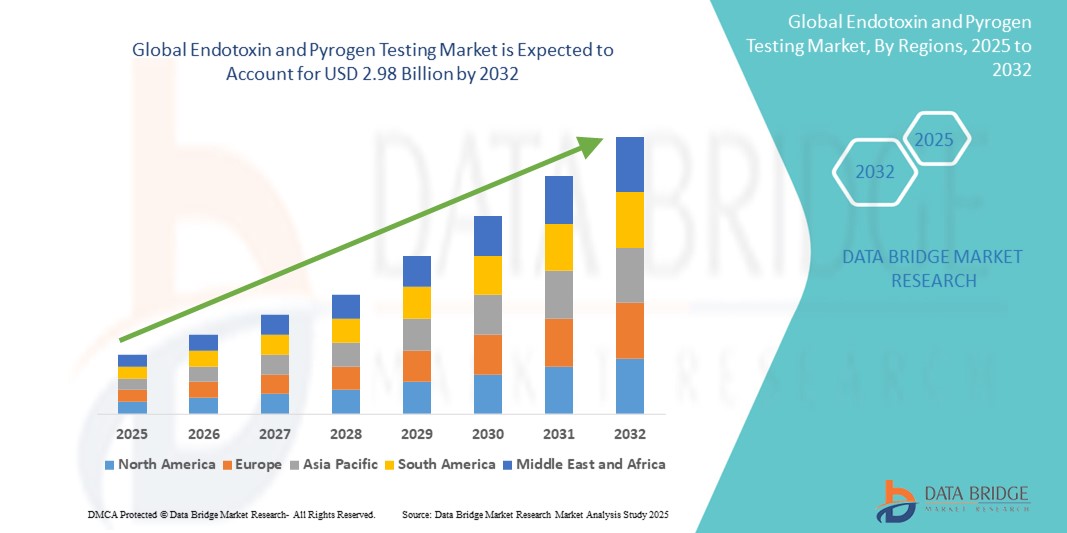
- The global endotoxin and pyrogen testing market size was valued at USD 1.27 billion in 2024 and is expected to reach USD 2.98 billion by 2032, at a CAGR of 11.3% during the forecast period
- The market growth is largely fueled by the increasing adoption and technological advancement in pharmaceutical quality control and biosafety processes, leading to greater digitalization and automation in pharmaceutical, biotechnology, and medical device manufacturing
- Furthermore, rising demand for accurate, rapid, and regulatory-compliant endotoxin and pyrogen detection methods is establishing Endotoxin and Pyrogen Testing as a critical component of modern pharmaceutical and medical device production workflows. These converging factors are accelerating the uptake of Endotoxin and Pyrogen Testing solutions, thereby significantly boosting the industry's growth
Endotoxin and Pyrogen Testing Market Analysis
- Endotoxin and Pyrogen Testing, offering critical biological safety assurance through the detection of harmful bacterial endotoxins and pyrogens in pharmaceutical and medical products, is increasingly vital in modern healthcare, biopharmaceutical, and device manufacturing due to its role in ensuring regulatory compliance and patient safety
- The escalating demand for Endotoxin and Pyrogen Testing is primarily fueled by the expanding biopharmaceutical industry, rising incidence of chronic diseases requiring injectable therapies, and heightened regulatory scrutiny surrounding contamination control in injectable drugs, vaccines, and implantable devices
- North America dominated the endotoxin and pyrogen testing market with the largest revenue share of 40.01% in 2024, characterized by early regulatory adoption, strong investment in biotechnology research, and the presence of major players offering advanced testing solutions. The U.S. captured 81% of the regional market, driven by a surge in biologics production and increasing adoption of automated and recombinant testing technologies across GMP-certified labs
- Asia-Pacific is projected to be the fastest-growing region in the endotoxin and pyrogen testing market during the forecast period, with a CAGR of 24.02% from 2025 to 2032, fueled by rapid industrialization, expanding pharmaceutical manufacturing capacity, and growing government support for quality control infrastructure in countries such as China, India, and Japan
- The detection kits and reagents segment dominated the endotoxin and pyrogen testing market with a share of 46.8% in 2024, owing to their widespread application, ease of use, and crucial role in routine quality assurance protocols across pharmaceutical and biotechnology companies
Report Scope and Endotoxin and Pyrogen Testing Market Segmentation
|
Attributes |
Endotoxin and Pyrogen Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Endotoxin and Pyrogen Testing Market Trends
“Growing Demand for Accuracy, Compliance, and Rapid Testing Solutions”
- A significant and accelerating trend in the Asia-Pacific endotoxin and pyrogen testing market is the increased emphasis on accuracy, rapid turnaround, and regulatory compliance in pharmaceutical and medical device manufacturing. This demand is driving the development and adoption of highly sensitive and standardized testing solutions
- For instance, leading players are introducing recombinant Factor C (rFC)-based endotoxin tests that eliminate the use of animal-derived reagents while ensuring precise and consistent results—addressing both ethical and regulatory concerns
- Technological advancements in automated systems are also enabling labs to streamline endotoxin detection processes, reduce manual intervention, and improve reproducibility. Automated plate readers and cartridge-based detection kits are gaining popularity for their user-friendly operation and minimal error margins
- The integration of advanced software with testing equipment allows for better traceability, real-time monitoring, and easier data reporting, all of which are critical for GMP compliance. This is especially relevant for pharmaceutical companies scaling production of sterile injectables, vaccines, and biologics
- Furthermore, the increasing collaboration between regional governments and pharmaceutical manufacturers to improve healthcare infrastructure—particularly post-COVID-19—has accelerated the need for reliable endotoxin and pyrogen testing protocols across the supply chain
- As a result, manufacturers are focusing on producing compact, automated, and highly sensitive test systems tailored to high-volume labs, which is reshaping expectations for efficiency, compliance, and scalability in quality control environments
Endotoxin and Pyrogen Testing Market Dynamics
Driver
“Growing Need Due to Rising Contamination Risks and Regulatory Stringency”
- The increasing prevalence of contamination risks in pharmaceutical, biotechnology, and medical device production, coupled with stricter regulatory frameworks, is a significant driver for the heightened demand for endotoxin and pyrogen testing solutions
- For instance, in April 2024, Onity, Inc. (Honeywell International, Inc.) announced advancements in biopharmaceutical safety technologies, aiming to integrate real-time detection sensors into aseptic manufacturing environments. Such developments by key players are expected to boost the growth of the endotoxin and pyrogen testing market across the Asia-Pacific region
- As companies prioritize patient safety and regulatory compliance, tests such as the Limulus Amebocyte Lysate (LAL) and Monocyte Activation Test (MAT) are increasingly being adopted for their ability to detect minute endotoxin levels in injectable drugs, vaccines, and implantable devices
- Furthermore, the growth of biologics and personalized medicines is fueling the need for reliable, high-throughput testing methods that can integrate seamlessly into quality assurance processes. Automated test platforms and rapid kits are becoming critical components of modern production lines
- The convenience of automation, reduced manual errors, and the capability to handle high-volume testing with accuracy are major factors propelling the adoption of these solutions across pharmaceutical companies, contract manufacturing organizations (CMOs), and research laboratories. The trend toward decentralizing manufacturing and expanding regional biotech hubs is further contributing to market growth in Asia-Pacific
Restraint/Challenge
“Concerns Regarding High Costs and Regulatory Harmonization”
- The high cost of recombinant or alternative endotoxin detection systems, along with the need for specialized instruments, can be a barrier for small and mid-sized companies in emerging economies within Asia-Pacific
- For instance, while traditional gel-clot LAL tests are relatively economical, more advanced kinetic chromogenic and turbidimetric methods, as well as MAT-based systems, come with significantly higher initial costs and training requirements
- Further, inconsistent regulatory adoption across countries in Asia-Pacific—such as varying acceptance levels of rFC or MAT methods—creates uncertainty among manufacturers looking to streamline their validation processes regionally
- Addressing these challenges through cost optimization, regional regulatory harmonization, and expanded training initiatives is crucial for boosting adoption. Leading players are increasingly offering bundled hardware-software solutions and technical support to mitigate these entry barriers and drive sustained market growth
Endotoxin and Pyrogen Testing Market Scope
The market is segmented on the basis of product & services, test type, type, product category, form, application, method, mode of purchase, end product, and end user.
• By Product and Services
On the basis of product and services, the endotoxin and pyrogen testing market is segmented into detection kits and reagents, instruments and systems, endotoxin and pyrogen testing services, and consumables and accessories. The detection kits and reagents segment dominated the market with the largest revenue share of 46.8% in 2024, driven by their high demand in pharmaceutical and biotechnology industries due to ease of use and reliable performance in endotoxin detection.
The endotoxin and pyrogen testing services segment is expected to grow at the highest CAGR of 11.2% from 2025 to 2032, due to increased outsourcing by pharma and biotech companies.
• By Test Type
On the basis of test type, the endotoxin and pyrogen testing market is segmented into Limulus Amoebocyte Lysate (LAL) Test, TAL Tests, Monocyte Activation Test (MAT), Recombinant Factor C (rFC) Assay, In-Vitro, and Rabbit Pyrogen Test. The LAL Test segment held the largest market share of 41.2% in 2024, owing to its regulatory acceptance and high sensitivity.
The Recombinant Factor C (rFC) Assay segment is projected to expand at a CAGR of 12.7% from 2025 to 2032, driven by animal-free testing demand and sustainability considerations.
• By Type
On the basis of type, the endotoxin and pyrogen testing market is segmented into pre-formed endotoxin and pyrogen testing, proendotoxin and pyrogen testing, and combined endotoxin and pyrogen testing. The combined endotoxin and pyrogen testing segment accounted for the largest market share of 38.5% in 2024, due to its capability to detect multiple contaminants efficiently.
The proendotoxin and pyrogen testing segment is expected to grow at a CAGR of 10.6% from 2025 to 2032, driven by demand for early-stage predictive testing.
• By Product Category
On the basis of product category, the endotoxin and pyrogen testing market is segmented into clean labelled ingredient and conventional. The conventional segment led the market with 58.1% share in 2024, as it includes widely used reagents and systems.
The clean labelled ingredient segment is projected to grow at a CAGR of 9.3% from 2025 to 2032, owing to transparency-driven regulatory shifts and consumer preferences.
• By Form
On the basis of form, the endotoxin and pyrogen testing market is segmented into powder and liquid. The liquid segment held the dominant share of 63.9% in 2024, due to ease of automation and direct usage.
The powder segment is expected to grow at a CAGR of 8.8% from 2025 to 2032, benefiting from longer shelf life and transportability.
• By Application
On the basis of application, the endotoxin and pyrogen testing market is segmented into pharmaceutical manufacturing, medical device manufacturing, raw materials production, and packaging manufacture. The pharmaceutical manufacturing segment accounted for the largest share of 49.5% in 2024, due to high sterility compliance needs.
The medical device manufacturing segment is projected to grow at a CAGR of 10.1% from 2025 to 2032, due to growing regulatory testing of implants and surgical equipment.
• By Method
On the basis of method, the endotoxin and pyrogen testing market is segmented into gel clot endotoxin and pyrogen test, chromogenic endotoxin and pyrogen test, and turbidimetric endotoxin and pyrogen test. The gel clot method dominated the market with 42.7% share in 2024, due to cost-effectiveness and regulatory approval.
The chromogenic method is anticipated to grow at the fastest CAGR of 11.4% from 2025 to 2032, supported by its quantitative accuracy and automation compatibility.
• By Mode of Purchase
On the basis of mode of purchase, the endotoxin and pyrogen testing market is segmented into large group, mid and small group, and individual. The large group segment held the largest market share of 55.2% in 2024, due to large-scale procurement by pharma and CDMOs.
The mid and small group segment is projected to grow at a CAGR of 9.9% from 2025 to 2032, with increasing demand from SMEs and academic institutions.
• By End Product
On the basis of end product, the endotoxin and pyrogen testing market is segmented into vaccines and/or CGT, biologics, injectables, and others. The biologics segment accounted for the largest share of 38.9% in 2024, driven by rising demand for monoclonal antibodies and biosimilars.
The vaccines and/or CGT segment is expected to grow at a CAGR of 12.1% from 2025 to 2032, due to increasing approvals in advanced therapies and vaccine development.
• By End User
On the basis of end user, the endotoxin and pyrogen testing market is segmented into pharmaceutical companies, biotechnology companies, biomedical companies, medical device companies, CROs, CMOs, and others. The pharmaceutical companies segment dominated the market with a share of 40.4% in 2024, due to consistent investment in quality control and compliance testing.
The contract research organizations (CROs) segment is expected to register the fastest CAGR of 11.6% from 2025 to 2032, fueled by increasing outsourcing and growing drug discovery pipelines.
Endotoxin and Pyrogen Testing Market Regional Analysis
- North America dominated the global endotoxin and pyrogen testing market with the largest revenue share of 40.01% in 2024, driven by increasing regulatory stringency, strong biopharmaceutical production pipelines, and widespread use of injectable drugs and biologics
- The region benefits from advanced healthcare infrastructure, a high volume of clinical trials, and a growing shift toward recombinant and animal-free testing methods. Leading companies in the U.S. and Canada are investing in automated endotoxin detection systems and sustainable testing solutions
- The growing preference for faster and compliant quality control protocols further strengthens the market presence in this region
U.S. Endotoxin and Pyrogen Testing Market Insight
The U.S. endotoxin and pyrogen testing market accounted for 83% of the North American market share in 2024. The country leads due to its large-scale biologics and vaccine manufacturing, heavy investment in pharmaceutical R&D, and swift adoption of recombinant factor C (rFC) assays and Monocyte Activation Tests (MAT). Regulatory alignment with the FDA’s push for alternatives to animal-based testing is further accelerating market expansion.
Europe Endotoxin and Pyrogen Testing Market Insight
The Europe endotoxin and pyrogen testing market is projected to grow at a notable CAGR during the forecast period due to rising awareness of product safety, strict EU pharmacopoeial requirements, and increased biologic product approvals. Countries such as Germany, the U.K., and France are emphasizing sustainable testing alternatives. Strong growth in contract manufacturing and R&D outsourcing is also contributing to market demand across drug development and medical device testing.
U.K. Endotoxin and Pyrogen Testing Market Insight
The U.K. endotoxin and pyrogen testing market is expected to grow steadily over the forecast period, supported by MHRA regulations aligned with global GMP standards, a mature biopharmaceutical sector, and growing investments in clinical research infrastructure. The demand for rapid endotoxin testing methods in sterility assurance and injectable drug production is a key growth driver.
Germany Endotoxin and Pyrogen Testing Market Insight
The Germany endotoxin and pyrogen testing market is expected to witness significant growth due to its robust pharmaceutical manufacturing base, automation in QC labs, and support for recombinant testing technologies. Regulatory adherence and a strong focus on product safety and efficiency are shaping procurement trends.
Asia-Pacific Endotoxin and Pyrogen Testing Market Insight
The Asia-Pacific endotoxin and pyrogen testing market is forecast to grow at the fastest CAGR of 24.02% (2025–2032), driven by growing pharmaceutical exports, rising demand for biologics, and government support for localized drug manufacturing in countries such as China, Japan, and India. The rapid expansion of clinical trial activity and greater awareness of contamination control standards are promoting the adoption of MAT and rFC assays in the region.
Japan Endotoxin and Pyrogen Testing Market Insight
The Japan endotoxin and pyrogen testing market is witnessing increased demand for pyrogen and endotoxin testing due to its high innovation in cell and gene therapies and its well-regulated pharmaceutical landscape. Regulatory bodies are encouraging non-animal-based testing alternatives, while the country’s aging population is increasing the need for injectable drugs.
China Endotoxin and Pyrogen Testing Market Insight
The China endotoxin and pyrogen testing market captured the largest market share within Asia-Pacific in 2024, driven by its vast pharmaceutical manufacturing capacity, strong government support for local biologics production, and increasing adoption of sustainable quality control solutions. The rise of domestic players offering cost-effective testing kits and services is further propelling market growth.
Endotoxin and Pyrogen Testing Market Share
The endotoxin and pyrogen testing industry is primarily led by well-established companies, including:
- Pall Corporation (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Charles River Laboratories (U.S.)
- Eurofins Scientific (Luxembourg)
- SGS Société Générale de Surveillance SA (Switzerland)
- Lonza (Switzerland)
- Merck KGaA (Germany)
- STERIS (Ireland)
- Sartorius AG (Germany)
- BIOMÉRIEUX (France)
- Ellab A/S (Denmark)
- ASSOCIATES OF CAPE COD, INC. (U.S.)
- WuXi AppTec (China)
- Microcoat Biotechnologie GmbH (Germany)
Latest Developments in Global Endotoxin and Pyrogen Testing Market
- In March 2024, Lonza Group announced the expansion of its endotoxin and pyrogen testing capabilities by launching a new recombinant factor C (rFC)-based assay kit. This development aligns with growing regulatory acceptance of animal-free testing methods and supports sustainability goals by reducing reliance on horseshoe crab blood
- In February 2024, Charles River Laboratories introduced enhanced Monocyte Activation Test (MAT) platforms through automation integration, aiming to improve the throughput and reproducibility of pyrogen detection in biologics and cell therapies. The innovation is targeted at streamlining workflows for pharmaceutical companies complying with stringent global regulations
- In January 2024, FUJIFILM Wako Chemicals USA Corporation upgraded its Limulus Amebocyte Lysate (LAL) reagent portfolio with increased sensitivity and reduced variability, enhancing the reliability of endotoxin testing in parenteral drugs and medical devices. The company also announced new distribution partnerships to expand its reach across Southeast Asia
- In December 2023, Associates of Cape Cod, Inc. (ACC) received additional approvals from Asian regulatory authorities for its PyroSmart NextGen rFC assay. The approval is expected to accelerate the adoption of sustainable, animal-free endotoxin testing methods across biopharma manufacturers in Japan, China, and South Korea
- In October 2023, Merck KGaA (MilliporeSigma) introduced a new high-throughput turbidimetric endotoxin testing solution designed for vaccine and biologics production. The launch is part of the company’s initiative to support faster lot-release testing while meeting evolving GMP standar
- In September 2023, Thermo Fisher Scientific revealed the development of an integrated LAL test automation platform in collaboration with global CDMOs. This solution combines LAL testing with real-time data capture, aiming to reduce human error, improve efficiency, and ensure audit-readiness in regulatory submissions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 COST ANALYSIS BREAKDOWN
8 TECHNONLOGY ROADMAP
9 INNOVATION TRACKER AND STRATEGIC ANALYSIS
9.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
9.1.1 JOINT VENTURES
9.1.2 MERGERS AND ACQUISITIONS
9.1.3 LICENSING AND PARTNERSHIP
9.1.4 TECHNOLOGY COLLABORATIONS
9.1.5 STRATEGIC DIVESTMENTS
9.2 NUMBER OF PRODUCTS IN DEVELOPMENT
9.3 STAGE OF DEVELOPMENT
9.4 TIMELINES AND MILESTONES
9.5 INNOVATION STRATEGIES AND METHODOLOGIES
9.6 RISK ASSESSMENT AND MITIGATION
9.7 FUTURE OUTLOOK
10 REGULATORY COMPLIANCE
10.1 REGULATORY AUTHORITIES
10.2 REGULATORY CLASSIFICATIONS
10.2.1 CLASS I
10.2.2 CLASS II
10.2.3 CLASS III
10.3 REGULATORY SUBMISSIONS
10.4 INTERNATIONAL HARMONIZATION
10.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
10.6 REGULATORY CHALLENGES AND STRATEGIES
11 REIMBURSEMENT FRAMEWORK
12 VALUE CHAIN ANALYSIS
13 HEALTHCARE ECONOMY
13.1 HEALTHCARE EXPENDITURE
13.2 CAPITAL EXPENDITURE
13.3 CAPEX TRENDS
13.4 CAPEX ALLOCATION
13.5 FUNDING SOURCES
13.6 INDUSTRY BENCHMARKS
13.7 GDP RATION IN OVERALL GDP
13.8 HEALTHCARE SYSTEM STRUCTURE
13.9 GOVERNMENT POLICIES
13.1 ECONOMIC DEVELOPMENT
14 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY PRODUCT AND SERVICES
14.1 OVERVIEW
14.2 DETECTION KITS & REAGENTS
14.2.1 LAL TEST REAGENTS
14.2.2 MYCOPLASMA DETECTION & REMOVAL
14.2.3 PCR MYCOPLASMA DETECTION KIT
14.2.4 MYCOPLASMA ELIMINATION COCKTAIL
14.2.5 OTHERS
14.3 INSTRUMENTS & SYSTEMS
14.3.1 SERIES TUBE READER
14.3.2 MICROPLATE READER
14.3.3 LOW ENDOTOXIN RECOVERY (LER)
14.3.4 ENDOTOXIN REMOVAL
14.3.5 LOW ENDOTOXIN RECOVERY (LER)
14.3.6 OTHERS
14.4 CONSUMABLES & ACCESSORIES
14.5 SOFTWARE AND SERVICES
14.6 OTHERS
15 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY TEST TYPE
15.1 OVERVIEW
15.2 LIMULUS AMOEBOCYTE LYSATE (LAL) TEST
15.3 MONOCYTE ACTIVATION TEST (MAT) TEST
15.4 RECOMBINANT FACTOR C (RFC) ASSAY
15.5 RABBIT PYROGEN TEST
16 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY TYPE
16.1 OVERVIEW
16.2 PRE-FORMED ENDOTOXIN AND PYROGEN TESTING
16.3 PROENDOTOXIN AND PYROGEN TESTING
16.4 COMBINE ENDOTOXIN AND PYROGEN TESTING
17 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY FORM
17.1 OVERVIEW
17.2 POWDER
17.3 LIQUID
18 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY PRODUCT CATEGORY
18.1 OVERVIEW
18.2 CLEAN LABELED INGREDIENT
18.3 CONVENTIONAL
19 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 PHARMACEUTICAL MANUFACTURING
19.3 MEDICAL DEVICE MANUFACTURING
19.4 RAW MATERIALS PRODUCTION
19.5 PACKAGING MANUFACTURE
20 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY METHOD
20.1 OVERVIEW
20.2 GEL CLOT ENDOTOXIN AND PYROGEN TEST
20.3 CHROMOGENIC ENDOTOXIN AND PYROGEN TEST
20.4 TURBIDIMETRIC ENDOTOXIN AND PYROGEN TEST
21 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY MODE OF PURCHASE
21.1 OVERVIEW
21.2 LARGE GROUP
21.3 MID AND SMALL GROUP
21.4 INDIVIDUAL
22 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY END PRODUCT
22.1 OVERVIEW
22.2 BIOLOGICS
22.3 VACCINES AND/ OR CGT
22.4 INJECTABLES
22.5 OTHERS
22.5.1 ENDOSCOPES
22.5.2 REUSABLE BIOMEDICAL DEVICES
22.5.3 OTHERS
23 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, BY END USER
23.1 OVERVIEW
23.2 PHARMACEUTICAL COMPANIES
23.3 BIOTECHNOLOGY COMPANIES
23.4 BIOMEDICAL COMPANIES
23.5 MEDICAL DEVICE COMPANIES
23.6 CONTRACT RESEARCH ORGANIZATION (CRO)
23.7 CONTRACT MANUFACTURING ORGANIZATION (CMO)
24 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, GEOGRAPHY
24.1 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 NORTH AMERICA
24.2.1 U.S.
24.2.2 CANADA
24.2.3 MEXICO
24.3 EUROPE
24.3.1 GERMANY
24.3.2 FRANCE
24.3.3 U.K.
24.3.4 ITALY
24.3.5 SPAIN
24.3.6 RUSSIA
24.3.7 BELGIUM
24.3.8 NETHERLANDS
24.3.9 SWITZERLAND
24.3.10 REST OF EUROPE
24.4 ASIA-PACIFIC
24.4.1 JAPAN
24.4.2 CHINA
24.4.3 SOUTH KOREA
24.4.4 INDIA
24.4.5 AUSTRALIA
24.4.6 SINGAPORE
24.4.7 MALAYSIA
24.4.8 REST OF ASIA-PACIFIC
24.5 SOUTH AMERICA
24.5.1 BRAZIL
24.5.2 ARGENTINA
24.5.3 REST OF SOUTH AMERICA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL ENDOTOXIN AND PYROGEN TESTING MARKET, COMPANY PROFILE
27.1 PALL EUROPE LIMITED (DANAHER CORPORATION)
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 THERMO FISHER SCIENTIFIC INC.
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 CHARLES RIVER LABORATORIES
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 EUROFINS SCIENTIFIC
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 SGS SOCIÉTÉ GÉNÉRALE DE SURVEILLANCE SA.
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 PACIFIC BIOLABS
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 LONZA
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 MERCK KGAA
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 STERIS
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 SARTORIUS AG
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 BIOMÉRIEUX
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 FUJIFILM WAKO PURE CHEMICAL CORPORATION
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ELLAB A/S.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 ASSOCIATES OF CAPE COD, INC (SEIKAGAKU CORPORATION)
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 WUXI APPTEC
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 GENSCRIPT
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 MICROCOAT BIOTECHNOLOGIE GMBH
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 SANQUIN
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 READING SCIENTIFIC SERVICES LTD
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 NANOCOMPOSIX
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 ZWISLER LABORATORIUM GMBH
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 NELSON LABORATORIES, LLC – A SOTERA HEALTH COMPANY
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 NORTH AMERICAN SCIENCE ASSOCIATES, LLC
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 PROMEGA CORPORATION
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 HYCULT BIOTECH INC.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 ALMAC GROUP
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 MAT BIOTECH
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 SOLVIAS
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 WICKHAM MICRO LIMITED
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 CREATIVE BIOLABS
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
28 RELATED REPORTS
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.