Global Endometrial Ablation Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
3.46 Billion
USD
5.56 Billion
2022
2030
USD
3.46 Billion
USD
5.56 Billion
2022
2030
| 2023 –2030 | |
| USD 3.46 Billion | |
| USD 5.56 Billion | |
|
|
|
|
Endometrial Ablation Devices Market Analysis and Size
According to the study titled "Radiofrequency endometrial ablation with a novel endometrial tip for the management of heavy menstrual bleeding and abnormal uterine bleeding: a prospective study," published in July 2020, the safety and efficacy of a radiofrequency ablation system with a new endometrial tip (RFA-EMT) for heavy menstrual bleeding (HMB) or abnormal uterine bleeding (AUB) were 97.4% and 100%, respectively. Thus, the increased safety and efficacy of the radiofrequency ablation system is expected to drive segment growth during the forecast period.
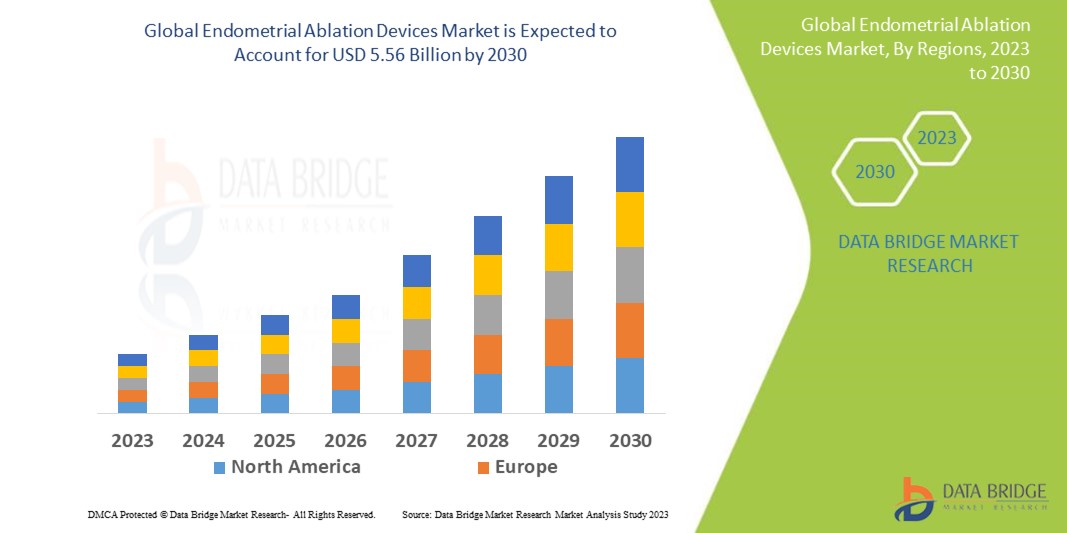
Data Bridge Market Research analyses that the endometrial ablation devices market which is USD 3.46 billion in 2022, is expected to reach USD 5.56 billion by 2030, at a CAGR of 6.1% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Endometrial Ablation Devices Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Device Type (Hysteroscopy devices, Thermal balloon ablators, Radiofrequency endometrial ablation devices, Hydrothermal ablators, Electrical ablators, Others), End-Use (Hospitals, Clinics, Ambulatory Surgical Centres, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
AEGEA Medical, Inc (U.S.), Boston Scientific Corporation (U.S.), CooperSurgical, Inc (U.S.), Hologic, Inc. (U.S.), IDOMAN-MED (Canada), Minerva Surgical, Inc (U.S.), Olympus Corporation (Japan), Omnitech Systems, Inc (U.S.) and Medtronic (Ireland) |
|
Market Opportunities |
|
Market Definition
Endometrial ablation is a medical procedure that allows for the removal of endometrium from the uterine wall. Endometrium ablation aids in the destruction of such thin layers to stop abnormal heavy uterine bleeding, and it is highly recommended when uterine bleeding is no longer controlled by medications.
Endometrial Ablation Devices Market Dynamics
Drivers
- Rise in the prevalence of gynaecological disorders
The rise in the prevalence of gynaecological disorders such as cervical cancer, menorrhagia, abnormal vaginal bleeding, and POCD (polycystic ovarian disease) in women is a major factor driving market growth. Cervical cancer is the fourth most common cancer in women worldwide, according to World Health Organization Updates in February 2022, with 604 000 new cases and 342 000 deaths by 2020. Low- and middle-income countries will account for nearly 90% of new cases and deaths globally by 2020. As a result, the increasing prevalence of gynaecological diseases is expected to drive market growth during the forecast period.
- Rising prevalence of endometrial cancer
The rising prevalence of endometrial cancer is expected to drive segment expansion. According to the American Cancer Society's 2022 update, more than 600,000 endometrial cancer survivors are in the United States. According to the same source, approximately 65,950 new cases of cancer of the uterine body (uterine body or corpus) are expected to be diagnosed in 2022. As a result of the increasing prevalence of endometrial cancer, demand for endometrial ablation devices is expected to rise.
Opportunities
- Rising technological developments
The increasing developments made by the key players in the ablation segment are also contributing to the market's growth. For instance, on Tuesday at the 50th Global Congress of the American Association of Gynecologic Laparoscopists (AAGL), Hologic, Inc. unveiled the NovaSure V5 global endometrial ablation (GEA) device in November 2021. .
Restraints/Challenges
- Risks associated with endometrial ablation
Risk associated with endometrial ablation is the most significant factor among others acting as restraints, and it will further challenge the growth of the endometrial ablation devices market during the forecast period.
This endometrial ablation devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the endometrial ablation devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Endometrial Ablation Devices Market
The healthcare sector has faced significant challenges as a result of the COVID-19 pandemic. All outpatient treatments were postponed or limited to reduce the risk of viral transmission, because most chronic therapies were deemed non-urgent during the COVID-19 pandemic. Endometriosis surgery is prohibited during the fourth, fifth, and sixth months of the COVID-19 pandemic, according to a study published in October 2021 titled "Endometriosis Surgery During the First Wave of the COVID-19 Pandemic: A Brazilian Single Institution Experience." Many endometriosis patients had postponed appointments, diagnostic tests, and elective therapeutic procedures. As a result, the decrease in endometrial surgeries during COVID-19 significantly impacted market growth. However, as endometrial surgeries resume and COVID-19 cases decline, the market is expected to return to pre-pandemic levels soon.
Recent Developments
- In 2022, CooperSurgical agreed to invest USD 875 million in Cook Medical's reproductive health portfolio, which includes fertility, obstetrics, gynaecology, and in vitro fertilization medical equipment.
- In 2022, Inovus Medical released HystAR, a new high-fidelity hysteroscopy simulator. The HystARTM is Inovus' third hysteroscopy simulator, joining the highly successful Bozzini Hysteroscopy line, which has been on the market for several years.
Global Endometrial Ablation Devices Market Scope
The endometrial ablation devices market is segmented on the basis of device type and end-use. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Device Type
- Hysteroscopy devices
- Thermal balloon ablators
- Radiofrequency endometrial ablation devices
- Hydrothermal ablators
- Electrical ablators
- Others
End-Use
- Hospitals
- Clinics
- Ambulatory Surgical Centres
- Others
Endometrial Ablation Devices Market Regional Analysis/Insights
The endometrial ablation devices market is analyzed and market size insights and trends are provided by country, device type and end-use as referenced above.
The countries covered in the endometrial ablation devices market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the endometrial ablation devices market due to increased prevalence of varicose veins, increased demand for minimally invasive treatment procedures.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 owing to the increased healthcare expenditure in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The endometrial ablation devices market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for endometrial ablation devices market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the endometrial ablation devices market. The data is available for historic period 2011-2021.
Competitive Landscape and Endometrial Ablation Devices Market Share Analysis
The endometrial ablation devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to endometrial ablation devices market.
Some of the major players operating in the endometrial ablation devices market are:
- AEGEA Medical, Inc (U.S.)
- Boston Scientific Corporation (U.S.)
- CooperSurgical, Inc (U.S.)
- Hologic, Inc. (U.S.)
- IDOMAN-MED (Canada)
- Minerva Surgical, Inc (U.S.)
- Olympus Corporation (Japan)
- Omnitech Systems, Inc (U.S.)
- Medtronic (Ireland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ENDOMETRIAL ABLATION DEVICES SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6. INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7. INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8. COST ANALYSIS BREAKDOWN
9. TECHNONLOGY ROADMAP
10. INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11. REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12. REIMBURSEMENT FRAMEWORK
13. OPPUTUNITY MAP ANALYSIS
14. VALUE CHAIN ANALYSIS
15. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY PRODUCT TYPE
15.1 OVERVIEW
15.2 SYSTEM/EQUIPMENT
15.2.1 BY TYPE
15.2.1.1. RADIOFREQUENCY ABLATION DEVICES
15.2.1.1.1. MONOPOLAR RADIOFREQUENCY ABLATION DEVICES
15.2.1.1.2. BIOPOLAR RADIOFREQUENCY ABLATION DEVICES
15.2.1.2. CRYOABLATION DEVICES
15.2.1.2.1. PROBE CRYOABLATION DEVICES
15.2.1.2.2. BALLOON CRYOABLATION DEVICES
15.2.1.3. THERMAL BALLOON ABLATION DEVICES
15.2.1.3.1. HYDROTHERMAL BALLOON ABLATION DEVICES
15.2.1.3.2. GAS-FILLED THERMAL BALLOON ABLATION DEVICES
15.2.1.4. HYDROTHERMAL ABLATION DEVICES
15.2.1.5. MICROWAVE ABLATION DEVICES
15.2.1.6. LASER ABLATION DEVICES
15.2.1.7. OTHERS
15.2.2 BY MODALITY
15.2.2.1. STANDALONE
15.2.2.2. PORTABLE
15.2.2.3. BENCHTOP
15.3 CONSUMABLES
15.3.1 ELECTRODE
15.3.1.1. ABLATION SINGLE ELECTRODE KITS
15.3.1.2. ABLATION CLUSTER ELECTRODE KITS
15.3.1.3. ABLATION MULTIPLE ELECTRODE KITS
15.3.2 ABLATION SYSTEM GROUNDING PAD
15.3.3 ENDOMETRIAL ABLATION PROBES
15.3.3.1. STERILE
15.3.3.2. NON-STERILE
15.3.4 CANNULA
15.3.5 NEEDLES
15.3.6 CATHETERS
15.3.7 OTHERS
15.4 OTHERS
16. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY PORTABILITY
16.1 OVERVIEW
16.2 STANDALONE
16.3 PORTABLE
16.4 BENCHTOP
17. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY SITE
17.1 OVERVIEW
17.2 IN OFFICE PROCEDURE SITE
17.3 FACILITY BASED PROCEDURE SITE
18. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY TECHNOLOGY
18.1 OVERVIEW
18.2 RADIOFREQUENCY ABLATION
18.3 CRYOABLATION
18.4 THERMAL BALLOON ABLATION
18.5 HYDROTHERMAL ABLATION
18.6 MICROWAVE ABLATION
18.7 LASER ABLATION
18.8 OTHERS
19. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY TECHNIQUE
19.1 OVERVIEW
19.2 ESECTOSCOPIC
19.2.1 SYSTEM/EQUIPMENT
19.2.2 CONSUMABLES
19.2.3 OTHERS
19.3 NON-RESECTOSCOPIC
19.3.1 SYSTEM/EQUIPMENT
19.3.2 CONSUMABLES
19.3.3 OTHERS
20. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY APPLICATION
20.1 OVERVIEW
20.2 ABNORMAL UTERINE BLEEDING (AUB)
20.2.1 SYSTEM/EQUIPMENT
20.2.2 CONSUMABLES
20.2.3 OTHERS
20.3 MENORRHAGIA (HEAVY MENSTRUAL BLEEDING)
20.3.1 SYSTEM/EQUIPMENT
20.3.2 CONSUMABLES
20.3.3 OTHERS
20.4 DYSMENORRHEA (PAINFUL MENSTRUATION)
20.4.1 SYSTEM/EQUIPMENT
20.4.2 CONSUMABLES
20.4.3 OTHERS
20.5 POLYPS
20.5.1 SYSTEM/EQUIPMENT
20.5.2 CONSUMABLES
20.5.3 OTHERS
20.6 FIBROIDS
20.6.1 SYSTEM/EQUIPMENT
20.6.2 CONSUMABLES
20.6.3 OTHERS
20.7 ADENOMYOSIS
20.7.1 SYSTEM/EQUIPMENT
20.7.2 CONSUMABLES
20.7.3 OTHERS
20.8 OTHERS
21. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY AGE GROUP
21.1 OVERVIEW
21.2 PRE-MENOPAUSAL
21.3 PERI-MENOPAUSAL
21.4 POST-MENOPAUSAL
22. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 BY TYPE
22.2.1.1. PRIVATE
22.2.1.2. PUBLIC
22.3 CLINICAL LABORATORIES
22.4 DIAGNOSTIC IMAGING CENTERS
22.5 INDEPENDENT TREATMENT CENTRES
22.6 CATHERIZATION LABORATORIES
22.7 AMBULATORY SURGICAL CENTERS
22.8 OTHERS
23. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDER
23.3 RETAIL SALES
23.4 OTHERS
24. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, SWOT AND DBMR ANALYSIS
25. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, BY REGION
GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
26.1 NORTH AMERICA
26.1.1 U.S.
26.1.2 CANADA
26.1.3 MEXICO
26.2 EUROPE
26.2.1 GERMANY
26.2.2 FRANCE
26.2.3 U.K.
26.2.4 ITALY
26.2.5 SPAIN
26.2.6 RUSSIA
26.2.7 TURKEY
26.2.8 BELGIUM
26.2.9 NETHERLANDS
26.2.10 SWITZERLAND
26.2.11 DENMARK
26.2.12 NORWAY
26.2.13 SWEDEN
26.2.14 FINLAND
26.2.15 POLAND
26.2.16 REST OF EUROPE
26.3 ASIA-PACIFIC
26.3.1 JAPAN
26.3.2 CHINA
26.3.3 SOUTH KOREA
26.3.4 INDIA
26.3.5 AUSTRALIA
26.3.6 SINGAPORE
26.3.7 THAILAND
26.3.8 MALAYSIA
26.3.9 INDONESIA
26.3.10 PHILIPPINES
26.3.11 VIETNAM
26.3.12 NEW ZEALAND
26.3.13 TAIWAN
26.3.14 REST OF ASIA-PACIFIC
26.4 SOUTH AMERICA
26.4.1 BRAZIL
26.4.2 ARGENTINA
26.4.3 REST OF SOUTH AMERICA
26.5 MIDDLE EAST AND AFRICA
26.5.1 SOUTH AFRICA
26.5.2 SAUDI ARABIA
26.5.3 UAE
26.5.4 EGYPT
26.5.5 ISRAEL
26.5.6 OMAN
26.5.7 QATAR
26.5.8 BAHRAIN
26.5.9 REST OF MIDDLE EAST AND AFRICA
26.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
27. GLOBAL ENDOMETRIAL ABLATION DEVICES MARKET, COMPANY PROFILE
27.1 HOLOGIC, INC.
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 COOPERSURGICAL
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 BOSTON SCIENTIFIC CORPORATION
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 IDOMAN-MED
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 OLYMPUS
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 OMNITECH SYSTEMS, INC
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 MEDTRONIC
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 VELDANA MEDICAL
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 CHANNEL MEDSYSTEMS, INC.
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.10 GYNESONICS
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 RF MEDICAL CO., LTD.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 LINA MEDICAL APS
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 JOHNSON & JOHNSON SERVICES, INC
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 OCON THERAPEUTICS
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 JUNE MEDICAL
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 KARL STORZ ENDOSCOPY-AMERICA, INC.
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 MGB BERLIN
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 GYNEX
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
28. RELATED REPORTS
29. CONCLUSION
30. QUESTIONNAIRE
31. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.