Global Dural Arteriovenous Fistulas Davf Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
2,080.44 Million
USD
3,537.57 Million
2022
2030
USD
2,080.44 Million
USD
3,537.57 Million
2022
2030
| 2023 –2030 | |
| USD 2,080.44 Million | |
| USD 3,537.57 Million | |
|
|
|
|
Dural Arteriovenous Fistulas (DAVF) Treatment Market Analysis and Size
The rise in the incidences of brain injury and neurological disorders among population across the globe, rise in investment in the research and development activities to enhance the overall course of diagnosis and treatment of dural arteriovenous fistulae (DAVF) and favorable reimbursement policies accelerate the growth and robust pipelines for development of newer treatment and significant investments in the development of advance technologies for the treatment methods further influence the growth of the global dural arteriovenous fistulas (DAVF) treatment market. In addition, improving healthcare infrastructure, increasing investment in biotechnology and pharmaceutical industries and surge in healthcare expenditure positively affect the dural arteriovenous fistulas (DAVF) treatment market.
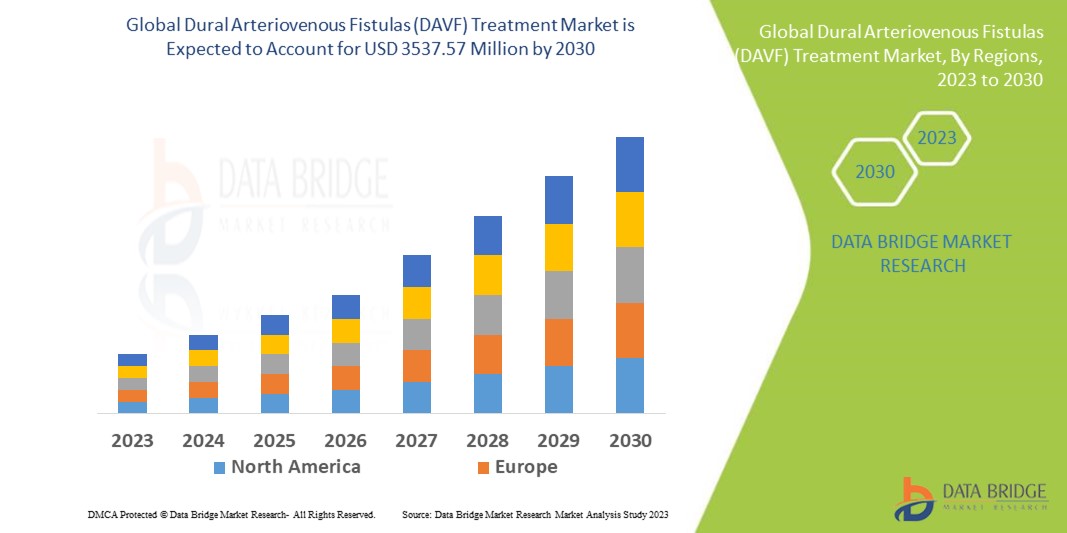
Data Bridge Market Research analyses that the global dural arteriovenous fistulas (DAVF) treatment market, which was USD 2080.44 million in 2022, is expected to reach USD 3537.57 million by 2030, and is expected to undergo a CAGR of 6.52% during the forecast period of 2023 to 2030. “Type 1” dominates the treatment segment of the global dural arteriovenous fistulas (DAVF) treatment market owing to the advancements in technology. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Dural Arteriovenous Fistulas (DAVF) Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Type I, Type II, Type III), Treatment (Surgery, Stereotactic Radiosurgery, Embolization), End User (Hospitals and Clinics, Ambulatory Surgical Centers) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Medtronic (Ireland), Stryker Corporation (U.S.), Johnson & Johnson Services, Inc. (U.S.), Penumbra, Inc. (U.S.), Terumo Corporation (Japan), Boston Scientific Corporation (U.S.), MicroVention, Inc. (U.S.), Balt Extrusion (France), Cook Medical (U.S.), Penumbra, Inc. (U.S.), Abbott Laboratories (U.S.), W.L. Gore & Associates (U.S.), DePuy Synthes (A subsidiary of Johnson & Johnson) (U.S.), Terumo Aortic (Belgium), Siemens Healthineers (Germany), Philips Healthcare (Netherlands), Elekta AB (Sweden), Varian Medical Systems (U.S.), Accuray Incorporated (U.S.), Brainlab AG (Germany) |
|
Market Opportunities |
|
Market Definition
Dural arteriovenous fistulae (DAVF) refer to a rare disease caused by abnormal connections between arteries and veins in a protective membrane on the outer layer of the brain and spine. These abnormal blood vessels tend to divert blood from the normal paths. Tissue downstream may not receive an adequate blood and oxygen supply if the volume of diverted blood flow is large. Headaches are among the most common symptoms among others depending on the location of fistulae.
Global Dural Arteriovenous Fistulas (DAVF) Treatment Market Dynamics
Drivers
- Increasing Prevalence of DAVF Treatment
The prevalence of dural arteriovenous fistulas (DAVF) is increasing globally. This condition, which involves abnormal connections between arteries and veins within the dura mater, is being diagnosed more frequently due to advancements in medical imaging technologies and increased awareness among healthcare professionals.
- Aging population Creating New Cases
Dural arteriovenous fistulas (DAVF) primarily affects older individuals, and as the global population continues to age, the number of cases is expected to rise. The elderly population is more prone to develop such vascular abnormalities, leading to a higher demand for effective treatment options.
- Technological Advancements in the Industry
Advances in medical technology, such as improved imaging techniques (such as digital subtraction angiography) and endovascular treatment methods (such as embolization), have significantly enhanced the diagnosis and treatment of dural arteriovenous fistulas (DAVF). These advancements have made the treatment more effective and less invasive, resulting in increased demand.
Opportunities
- Growing Awareness and Education
Greater awareness about dural arteriovenous fistulas (DAVF) among healthcare professionals and patients has led to early detection and diagnosis. Educational efforts by medical societies and patient advocacy groups have played a crucial role in disseminating information about the condition, its symptoms, and available treatment options.
- Rise in the Healthcare Expenditure
Increased healthcare spending, particularly in developed countries, enables patients to access advanced medical treatments, including dural arteriovenous fistulas (DAVF) treatment. The availability of financial resources facilitates the adoption of innovative treatment options and contributes to the growth of the market.
Restraints/Challenges
- High Cost of Treatment
The treatment of Dural Arteriovenous Fistulas (DAVF) can involve complex procedures, advanced imaging techniques, and specialized medical devices. These factors contribute to the overall cost of treatment, which can be a significant barrier for patients, particularly in regions with limited healthcare resources or inadequate insurance coverage.
-
Lack of Specialized Expertise
The treatment of dural arteriovenous fistulas (DAVF) often requires specialized expertise in neurovascular interventions. However, there may be a shortage of healthcare professionals with the necessary skills and experience to effectively diagnose and treat DAVF, particularly in certain regions. This can limit access to appropriate treatment options.
This global dural arteriovenous fistulas (DAVF) treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global dural arteriovenous fistulas (DAVF) treatment market Contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In April 2021, Medtronic, a global medical technology company, has been involved in the development of innovative endovascular devices for the treatment of vascular conditions, including DAVF. They have introduced products such as the Pipeline Flex Embolization Device, which is designed to divert blood flow away from the DAVF, and the Excelsior SL-10 Microcatheter, which aids in the delivery of embolic agents
Global Dural Arteriovenous Fistulas (DAVF) Treatment Market Scope
The global dural arteriovenous fistulas (DAVF) treatment market is segmented on the basis type, treatment and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Type I
- Type II
- Type III
Treatment
- Surgery
- Stereotactic Radiosurgery
- Embolization
End User
- Hospitals and Clinics
- Ambulatory Surgical Centers
Global Dural Arteriovenous Fistulas (DAVF) Treatment Market Regional Analysis/Insights
The global dural arteriovenous fistulas (DAVF) treatment market is analysed and market size insights and trends are provided by country, basis of type, treatment and end user as referenced above.
The countries covered in the global dural arteriovenous fistulas (DAVF) treatment market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the global dural arteriovenous fistulas (DAVF) treatment market due to the strong base of healthcare facilities, strong presence of major players in the market, rising demand of the dural arteriovenous fistulas (DAVF) treatment products and rising number of research activities in this region.
Asia-Pacific is expected to witness significant growth in the global dural arteriovenous fistulas (DAVF) treatment market during the forecast period of 2023 to 2030 due to the increase in government initiatives to promote awareness, rise in medical tourism, growing research activities in the region, availability of massive untapped markets, large population pool, and the growing demand for quality healthcare in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The global dural arteriovenous fistulas (DAVF) treatment market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for global dural arteriovenous fistulas (DAVF) treatment market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the global dural arteriovenous fistulas (DAVF) treatment market The data is available for historic period 2015-2020.
Competitive Landscape and Global Dural Arteriovenous Fistulas (DAVF) Treatment Market Share Analysis
The global dural arteriovenous fistulas (DAVF) treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related global dural arteriovenous fistulas (DAVF) treatment market.
Some of the major players operating in the global dural arteriovenous fistulas (DAVF) treatment market are:
- Medtronic (Ireland)
- Stryker Corporation (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Penumbra, Inc. (U.S.)
- Terumo Corporation (Japan)
- Boston Scientific Corporation (U.S.)
- MicroVention, Inc. (U.S.)
- Balt Extrusion (France)
- Cook Medical (U.S.)
- Penumbra, Inc. (U.S.)
- Abbott Laboratories (U.S.)
- W.L. Gore & Associates (U.S.)
- DePuy Synthes (A subsidiary of Johnson & Johnson) (U.S.)
- Terumo Aortic (Belgium)
- Siemens Healthineers (Germany)
- Philips Healthcare (Netherlands)
- Elekta AB (Sweden)
- Varian Medical Systems (U.S.)
- Accuray Incorporated (U.S.)
- Brainlab AG (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.