Global Clinical Trials Market, By Phase (Phase I, Phase II, Phase III, Phase IV), Indication (Autoimmune/Inflammation, Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular, Others), Design (Interventional, Treatment Studies, Observational Studies, Expanded Access), End User (Hospital, Laboratories, Clinics) – Industry Trends and Forecast to 2030.
Clinical Trials Market Analysis and Size
The growing demand for clinical trial in developing countries, growing geriatric population, globalization of clinical trials, technological evolution are the significant factors responsible for driving the growth of the clinical trials market. The leveraging online resources to increase patient recruitment rates in clinical trials is also projected to boost the market’s growth. In addition, the increasing occurrences of chronic diseases coupled with globalizing drug development activities also heighten the overall growth of the market.
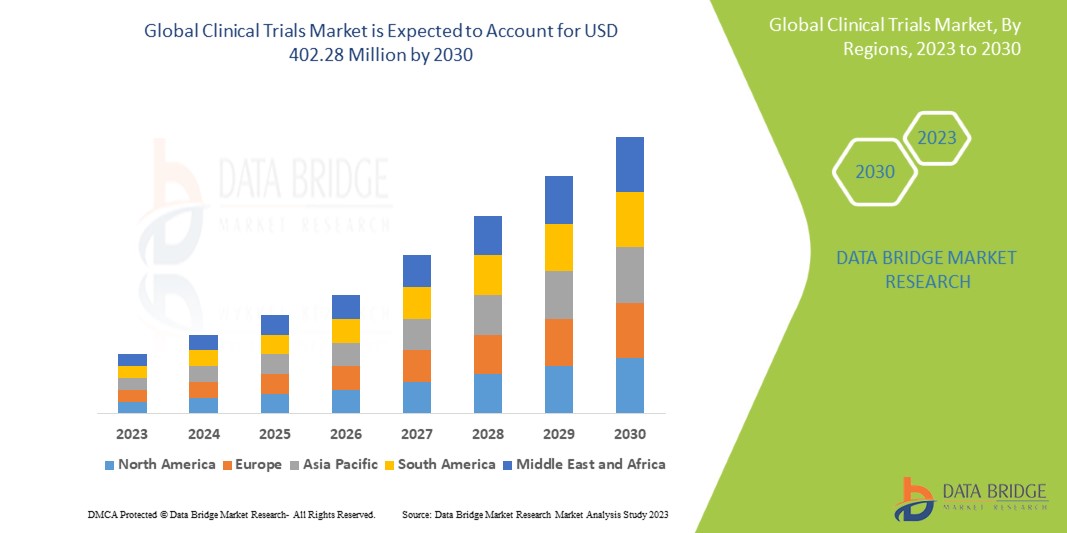
Data Bridge Market Research analyzes that the global clinical trials market which was USD 269.18 million in 2022, is likely to reach USD 402.28 million by 2030, and is expected to undergo a CAGR of 5.15% during the forecast period. “Laboratories" dominates the end segment of the clinical trials market due to increase in the number of research and development activities. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
By Phase (Phase I, Phase II, Phase III, Phase IV), Indication (Autoimmune/Inflammation, Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular, Others), Design (Interventional, Treatment Studies, Observational Studies, Expanded Access), End User (Hospital, Laboratories, Clinics)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, Rest of the Middle East & Africa
|
|
Market Players Covered
|
Clinipace (US.), Laboratory Corporation of America Holdings (LabCorp) (U.S.), Eli Lilly and Company (U.S.), ICON Plc. (Ireland), Novo Nordisk A/S (Denmark), Parexel International Corporation (U.S.), Pfizer Inc. (U.S.), PPD, Inc. (U.S.), IQVIA (U.S.), Sanofi (France), F. Hoffmann-La Roche Ltd (Switzerland), Alcami Corporation, Inc. (U.S.), Accell Clinical Research LLC (U.S.), Congenix LLP (U.S.), Labcorp Drug Development (U.S.), Ecron Acunova (India), Medpace (U.S.), LUMITOS AG (Germany), ICON plc (Ireland), SIRO Clinpharm Private Limited (India)
|
|
Market Opportunities
|
|
Market Definition
Clinical trials are basically the research studies performed by researchers in people that are aimed to find out a new treatment, like a new drug or diet or medical device. It is also used to find out whether it is safe and effective in people.
Clinical Trials Market Dynamics
Drivers
- Advancements in medical research
Increasing focus on developing novel therapies and treatments for various diseases drives the demand for clinical trials. Advances in genomics and personalized medicine also contribute to the growth.
- Rising prevalence of diseases
The growing incidence of chronic diseases like cancer, diabetes, and cardiovascular disorders necessitates extensive clinical trials for innovative drugs and therapies.
- Technological advancements
Integration of technologies like big data analytics, AI, and IoT in clinical trials streamlines processes, enhances data accuracy, and reduces costs, thereby driving market growth.
Opportunities
- Real-world evidence (RWE) studies
The integration of real-world data into clinical trials provides valuable insights into drug effectiveness and safety, opening avenues for more pragmatic and efficient trials
- Patient-centric trials
Emphasizing patient experience and convenience through methods like virtual trials and home healthcare services can enhance participation rates and data accuracy.
Restraints/Challenges
- Stringent regulatory procedures
Complex regulatory processes and the need for compliance with diverse guidelines in different countries pose challenges for sponsors, leading to delays and increased costs.
- Data security and privacy concerns
With the rise in digitalization, ensuring the security and privacy of patient data in clinical trials is a significant challenge. Adhering to data protection regulations adds complexity.
- Patient recruitment and retention
Identifying suitable participants and retaining them throughout the trial period is challenging. This affects the timeline and success of the trials.
This clinical trials market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the clinical trials market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Clinical Trials Market Scope
Clinical trails market is segmented on the basis of phase, indication, design, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries, and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Indication
- Autoimmune/inflammation
- Pain management
- Oncology
- Cns condition
- Diabetes
- Obesity
- Cardiovascular
- Others
Design
- Interventional
- Treatment studies
- Observational studies
- Expanded access
End-Users
- Hospitals
- Laboratories
- Clinics
Clinical Trials Market Regional Analysis/Insights
Clinical trials market is analyzed and market size insights and trends are provided by country, phase, indication, design and end user as referenced above.
The countries covered in the clinical trials market report is U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America, South Africa, Saudi Arabia, U.A.E, Egypt, Israel, rest of Middle East & Africa.
North America is expected to dominate the market due to increasing research and development and increasing adoption of new technologies in clinical research.
Asia-Pacific is expected to show fastest growth during the forecast period of 2023 to 2030 due to expected to show a rapid and lucrative growth rate in the forecast period owing to the increasing availability of large patient pool facilitating easy recruitment of candidates.
The country section of the report also provides individual market impacting factors and domestic regulation changes that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, and case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The clinical trials market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for clinical trials market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on clinical trials market. The data is available for historic period 2010-2020.
Competitive Landscape and Clinical Trials Market Share Analysis
The clinical trials market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to clinical trials market.
Some of the major players operating in the global clinical trials market are:
- Clinipace (U.S.)
- Laboratory Corporation of America Holdings (LabCorp) (U.S.)
- Eli Lilly and Company (U.S.)
- ICON Plc. (Ireland)
- Novo Nordisk A/S (Denmark)
- Parexel International Corporation (U.S.)
- Pfizer Inc. (U.S.)
- PPD, Inc. (U.S.)
- IQVIA (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Alcami Corporation, Inc. (U.S.)
- Accell Clinical Research LLC (U.S.)
- Congenix LLP (U.S.)
- Labcorp Drug Development (U.S.)
- Ecron Acunova (India)
- Medpace (U.S.)
- LUMITOS AG (Germany)
- ICON plc (Ireland)
- SIRO Clapham Private Limited (India)
SKU-