Global Clinical Trials Market
Market Size in USD Billion
CAGR :
% 
 USD
297.62 Billion
USD
444.77 Billion
2024
2032
USD
297.62 Billion
USD
444.77 Billion
2024
2032
| 2025 –2032 | |
| USD 297.62 Billion | |
| USD 444.77 Billion | |
|
|
|
|
Clinical Trial Market Size
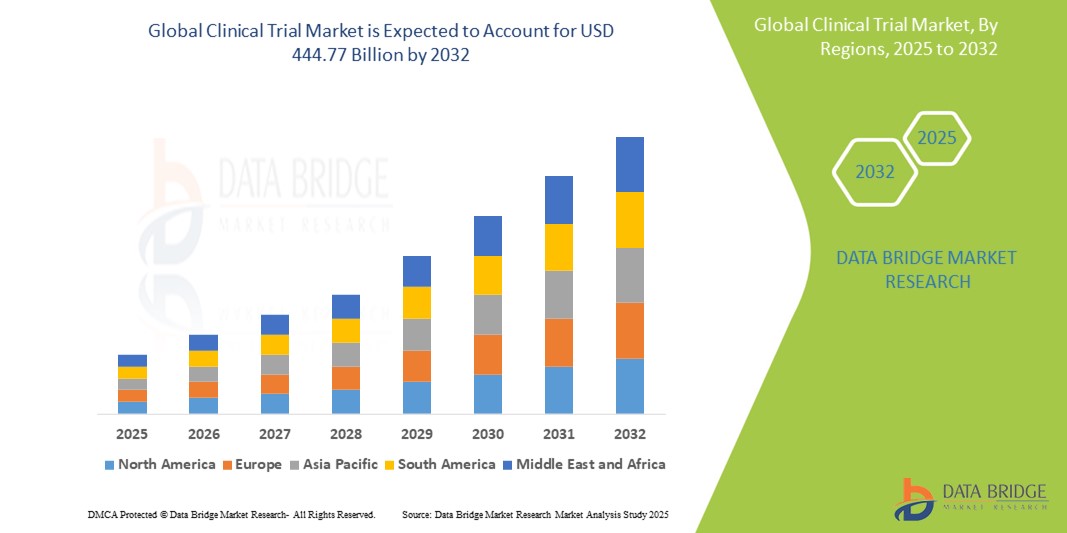
- The global Clinical Trial market was valued at USD 297.62 million in 2024 and is expected to reach USD 444.77 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 5.15 % primarily driven by the increasing demand for innovative therapies, advancements in clinical research technologies, and the rise in clinical trial outsourcing
- This growth is driven by factors such as the growing complexity of clinical trials, increasing number of clinical trials, expanding adoption of decentralized trials, and the rising need for cost-effective and faster trial processes
Clinical Trial Market Analysis
- A clinical trial is a research study involving human participants to assess the safety, efficacy, and potential side effects of new medical treatments or interventions
- The global clinical trial market is expanding rapidly, driven by an increasing focus on developing novel therapies and improving patient care with new treatments and medications, especially in areas such as oncology and rare diseases
- For instance, the development of CAR T-cell therapies for cancer treatment has significantly accelerated clinical trials in oncology
- Technological advancements, such as the use of artificial intelligence and machine learning for data analysis, have significantly improved the efficiency and accuracy of clinical trials
- For instance, the use of AI in patient recruitment for clinical trials, with companies such as IBM Watson Health leading efforts to streamline the process and ensure faster participant matching
- The number of clinical trials conducted globally has increased, with clinical trials for COVID-19 vaccines being a prime instance of how the industry can quickly adapt to meet urgent public health needs. The rapid clinical trial process behind the Pfizer and Moderna COVID-19 vaccines highlights the sector’s ability to accelerate development under pressure
- Outsourcing of clinical trials to contract research organizations (CROs) is becoming more common, helping pharmaceutical companies reduce costs and access specialized expertise. A real-time instance includes the collaboration between Novartis and Covance for global clinical trials, where Covance provided specialized services in managing large-scale, international trials
- The integration of decentralized trials, which allow patients to participate from home through digital platforms, has been accelerated, particularly in the wake of the pandemic
- For instance, the clinical trials for AstraZeneca’s COVID-19 vaccine, which included decentralized trial models to increase participation and maintain safety protocols
Report Scope and Clinical Trial Market Segmentation
|
Attributes |
Clinical Trial Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Clinical Trial Market Trends
“Growing Adoption of Decentralized Clinical Trials”
- Decentralized clinical trials enable patients to participate from home or local healthcare facilities, reducing the need for frequent visits to central research sites, which improves convenience for participants
- For instance, Pfizer's remote trials during the COVID-19 pandemic allowed patients to participate in vaccine studies without needing to visit the trial site
- These trials leverage technology such as telemedicine, wearable devices, and digital platforms to monitor patients, collect data, and maintain communication, allowing real-time tracking of patient health
- For instance, Eli Lilly used wearable devices to monitor patients remotely in its clinical trials for diabetes medication
- The COVID-19 pandemic accelerated the adoption of decentralized clinical trials as the healthcare industry adjusted to ensure continuity
- For instance, Moderna conducted remote trials for their COVID-19 vaccine development, using telehealth and at-home monitoring to track vaccine recipients while maintaining safety and reducing exposure risks
- Decentralized trials have increased patient access, especially in rural or underserved areas, by eliminating the barriers of traveling long distances to clinical sites
- For instance, AstraZeneca’s COVID-19 vaccine trials utilized virtual consultations and home delivery of medications, allowing patients in remote locations to stay engaged in the trial
- Pharmaceutical companies and contract research organizations are now adopting hybrid models, combining traditional site-based trials with decentralized methods
- For instance, Novartis has incorporated decentralized elements into its ongoing global trials for oncology treatments, allowing patients in remote areas to participate while still adhering to trial protocols
Clinical Trial Market Dynamics
Driver
“Increasing Demand for New and Innovative Therapies”
- The increasing demand for new and innovative therapies, especially in oncology, rare diseases, and chronic conditions, is driving the clinical trial market
- For instance, the rise of personalized cancer treatments such as CAR T-cell therapy has spurred numerous trials to test their efficacy and safety
- Pharmaceutical companies and research institutions are focusing on targeted treatments and precision medicine, which require robust clinical trials
- For instance, the development of gene therapies, such as those for spinal muscular atrophy, which have undergone extensive clinical trials to ensure their effectiveness
- Breakthrough treatments such as immune checkpoint inhibitors for cancer, including drugs such as Keytruda and Opdivo, have accelerated the demand for clinical trials to refine and expand their applications, with many trials now investigating their use in various cancer types
- The rapid development of COVID-19 vaccines, including those from Pfizer and Moderna, highlighted the urgent need for fast-tracked clinical trials, showcasing how clinical research can quickly adapt to public health needs and expedite the delivery of life-saving treatments
- The increasing prevalence of chronic diseases and aging populations is contributing to the rise in clinical trials
- For instance, the growing global incidence of diabetes has led to numerous trials exploring new treatments and technologies to improve patient outcomes
Opportunity
“Increasing Demand for Clinical Trials in Emerging Markets”
- Increasing demand for clinical trials in emerging markets such as India and China, where large, diverse patient populations offer faster recruitment and varied testing conditions
- For instance, in India, trials for vaccines and cancer treatments have been expanding due to the availability of a large and diverse patient pool
- Growing prevalence of chronic diseases and aging populations globally, creating a need for more therapies and expanding clinical trial opportunities in areas such as oncology and diabetes. The rise in diabetes cases has led to increased clinical trials testing new insulin formulations and management therapies in countries such as the U.S. and Europe
- Technological advancements such as digital platforms and virtual trials, streamlining recruitment, data collection, and monitoring while reducing trial costs
- For instance, platforms such as science 37 have enabled remote clinical trials, allowing patients to participate in trials from their homes and reducing the need for travel
- Expanding regulatory flexibility in countries such as Brazil and South Korea, allowing for faster trial approvals and encouraging international pharmaceutical companies to conduct research in these regions. In Brazil, the regulatory body, ANVISA, has simplified the approval process for clinical trials, making it a key destination for clinical research in Latin America
- Increased focus on personalized medicine, leading to the development of precision trials tailored to specific patient subgroups, such as the rise of gene therapies for rare diseases
- For instance, the clinical trials for gene therapy treatments such as Zolgensma for spinal muscular atrophy, which is tailored to specific genetic markers in affected children
Restraint/Challenge
“Complexity with Conducting Clinical Trials”
- Regulatory challenges arise from differing guidelines across countries, complicating the approval process
- For instance, the recent delays in trials for COVID-19 vaccines due to varying regulations from the U.S. FDA and the EMA in Europe demonstrated how complex international compliance can be
- Patient recruitment and retention are often hindered by limited awareness and accessibility, particularly in underserved regions
- For instance, clinical trials for rare diseases such as Duchenne muscular dystrophy face challenges in recruiting enough eligible participants, delaying research and development
- Data management becomes increasingly difficult as trials generate massive volumes of information. In multicenter trials, ensuring the accuracy and consistency of data collection, such as in oncology trials, requires advanced technology to prevent errors, as was evident in the AstraZeneca vaccine trial where data inconsistencies caused brief setbacks
- Cost and time constraints remain a major issue in clinical trials, especially those involving long durations or high patient follow-up. The cost of running large-scale trials such as the ones for Alzheimer's drugs can exceed millions of dollars, and delays are common, as seen in the trial delays for the drug aducanumab
- Ethical concerns surrounding patient safety and informed consent are critical, especially when vulnerable populations are involved. Clinical trials for gene therapies, such as those for spinal muscular atrophy, require careful ethical consideration to ensure that patients fully understand the risks, as seen with the approval process for Zolgensma
Clinical Trial Market Scope
The market is segmented on the basis of phase, indication, design, and end user
|
Segmentation |
Sub-Segmentation |
|
By Phase |
|
|
By Indication |
|
|
By Design |
|
|
By End User |
|
Clinical Trial Market Regional Analysis
“North America is the Dominant Region in the Clinical Trial Market”
- North America dominates the Clinical Trial market, primarily due to the U.S. strong healthcare system with advanced technology, providing a solid foundation for clinical trials
- The region is home to many global pharmaceutical and biotech companies, driving innovation and clinical research
- North America benefits from clear and supportive regulatory processes that facilitate the approval and execution of clinical trials
- Continued funding for clinical research, along with a large, diverse patient pool, accelerates market growth and enhances North America’s dominance
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is the fastest-growing market in Clinical Trial, driven by the need for innovative treatments and drugs in a rapidly expanding healthcare market
- Attractive destinations for clinical trials, particularly in China, India, and Japan, due to vast and diverse populations and lower costs compared to Western markets
- Improvements in healthcare infrastructure and the rise in the number of clinical research sites contributing to the region's rapid growth
- More favorable regulatory environment, supporting the efficient execution of clinical trials in the region
- Ongoing investments in the healthcare sector and a growing number of experienced professionals, positioning Asia-Pacific as the fastest-growing region in clinical trials
Clinical Trial Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Clinipace (U.S.)
- Laboratory Corporation of America Holdings (LabCorp) (U.S.)
- Eli Lilly and Company (U.S.)
- ICON Plc. (Ireland)
- Novo Nordisk A/S (Denmark)
- Parexel International Corporation (U.S.)
- Pfizer Inc. (U.S.)
- PPD, Inc. (U.S.)
- IQVIA (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Alcami Corporation, Inc. (U.S.)
- Accell Clinical Research LLC (U.S.)
- Congenix LLP (U. K.)
- Labcorp Drug Development (U.S.)
- Ecron Acunova (India)
- Medpace (U.S.)
- LUMITOS AG (Germany)
- ICON plc (Ireland)
- SIRO Clapham Private Limited (India)
Latest Developments in Global Clinical Trial Market
- In February 2025, Novotech partnered with Wonju Severance Christian Hospital to drive clinical research excellence in South Korea. This collaboration aims to enhance clinical trial capabilities by leveraging the hospital’s advanced medical infrastructure and expertise. The partnership will focus on improving clinical research services and expanding clinical trial opportunities in Korea. The development is expected to increase the speed and efficiency of clinical trials, benefiting both local and international pharmaceutical companies by providing a stronger, more reliable research environment. The impact on the market will be significant, as it positions South Korea as a more attractive destination for global clinical trials, potentially accelerating the growth of the clinical research market in the Asia-Pacific region
- In January 2025, ICON plc announced the development of its AI-powered portfolio of tools to enhance clinical trial efficiencies. The company’s new AI solutions are designed to streamline various aspects of clinical trials, including patient recruitment, data analysis, and operational management. This advancement will enable faster trial timelines, reduced costs, and improved decision-making throughout the trial process. The benefits include optimized resource utilization, better patient targeting, and enhanced accuracy in trial outcomes. The impact on the market will be significant, as these AI-driven tools are set to transform the clinical trial landscape, making it more efficient, cost-effective, and scalable for global pharmaceutical companies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.