Global Cardiac Bio Implant Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
55.08 Billion
USD
119.99 Billion
2024
2032
USD
55.08 Billion
USD
119.99 Billion
2024
2032
| 2025 –2032 | |
| USD 55.08 Billion | |
| USD 119.99 Billion | |
|
|
|
|
Cardiac Bio Implant Devices Market Analysis
The cardiac bio implant devices market is a rapidly growing sector within the global healthcare industry, driven by the increasing prevalence of cardiovascular diseases, advancements in implant technologies, and the rising demand for personalized treatment options. These devices, including pacemakers, defibrillators, heart valves, and bio-resorbable stents, offer innovative solutions for managing and treating various heart conditions. Recent developments in the market focus on improving the biocompatibility, longevity, and functionality of these devices, with a trend towards minimally invasive procedures and remote monitoring capabilities. In addition, innovations in regenerative medicine, such as stem cell-based implants, are expected to transform the market in the coming years. The market is experiencing robust growth, particularly in developed regions, due to advancements in healthcare infrastructure and increasing adoption of bio implant devices. However, challenges related to high costs, regulatory approvals, and potential risks associated with long-term use of bio-implants continue to impact market dynamics.
Cardiac Bio Implant Devices Market Size
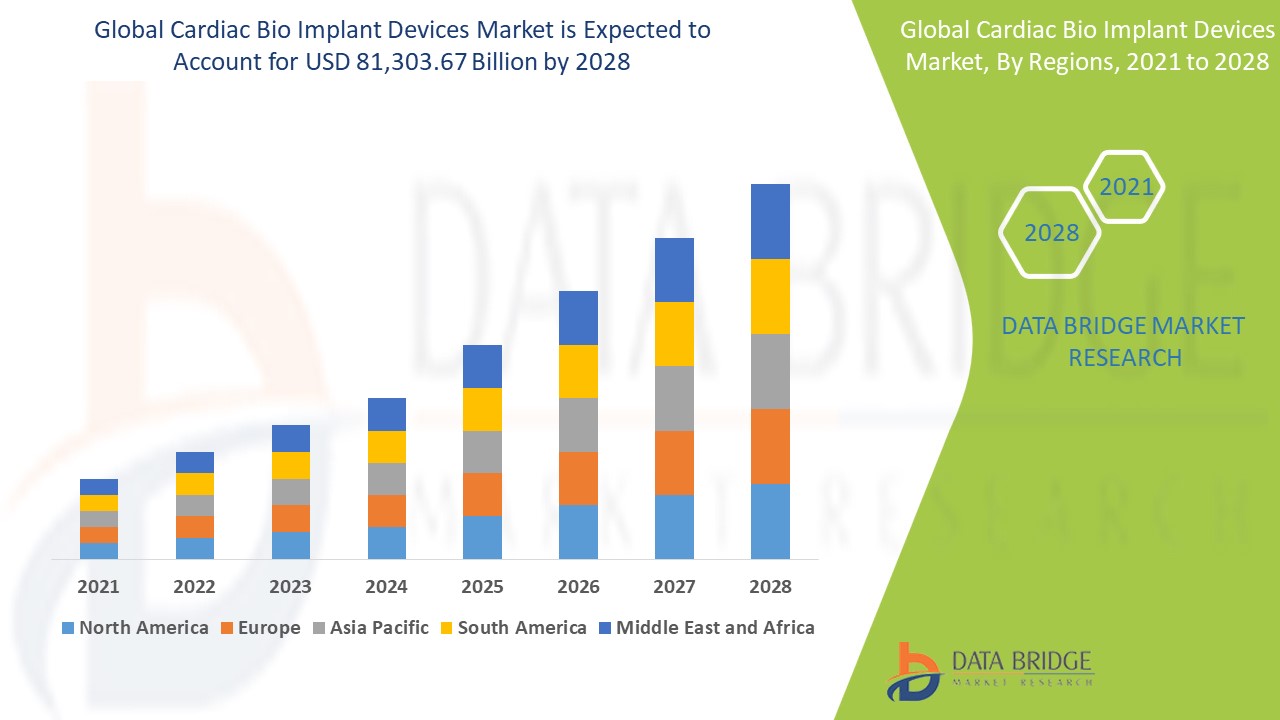
The global cardiac bio implant devices market size was valued at USD 55.08 billion in 2024 and is projected to reach USD 119.99 billion by 2032, with a CAGR of 10.22% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Cardiac Bio Implant Devices Market Trends
“Innovations in Bio-Compatible Materials”
The cardiac bio implant devices market is witnessing significant growth, fueled by rising cardiovascular disease prevalence and technological advancements. Innovations in bio-compatible materials, such as polymers and bio-resorbable stents, are enhancing the effectiveness and safety of these implants. A key trend in the market is the integration of smart technologies, including remote monitoring and data-driven solutions, which allow healthcare providers to track patients' heart health in real time, improving treatment outcomes. Moreover, there is increasing demand for minimally invasive procedures, driving the development of smaller, more efficient implants. These trends reflect a broader shift towards personalized, patient-centric care in the cardiovascular space, propelling the market's expansion globally.
Report Scope and Cardiac Bio Implant Devices Market Segmentation
|
Attributes |
Cardiac Bio Implant Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Abbott (U.S.), Edwards Lifesciences Corporation (U.S.), Johnson & Johnson Services, Inc. (U.S.), LivaNova PLC (U.K.), Medtronic (U.S.), Boston Scientific Corporation (U.S.), MicroPort Scientific Corporation (China), Mayo Foundation for Medical Education and Research (MFMER) (U.S.), ClearPoint Neuro, Inc. (U.S.), Imricor (U.S.), Biotronik (Germany), ELESTIM-CARDIO (Russia), QualiMed (Germany), MEDICO S.R.L. (Italy), Lepu Medical Technology (Beijing) Co., Ltd. (China), W. L. Gore & Associates, Inc. (U.S.), LifeNet Health (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Cardiac Bio Implant Devices Market Definition
Cardiac bio implant devices are medical devices implanted in the human heart or cardiovascular system to treat or manage heart-related conditions. These devices are made from bio-compatible materials designed to interact safely with human tissues. Common types include pacemakers, defibrillators, heart valves, and bio-resorbable stents. These implants help regulate heart rhythms, support heart function, and improve blood flow, often used in patients with cardiovascular diseases such as arrhythmias, heart failure, or coronary artery disease.
Cardiac Bio Implant Devices Market Dynamics
Drivers
- Rising Prevalence of Cardiovascular Diseases
The rising incidence of heart-related conditions, including heart failure, arrhythmias, and coronary artery disease, is significantly driving the demand for cardiac bio implant devices. As the global population ages and lifestyle factors contribute to an increased prevalence of cardiovascular diseases, more patients require advanced medical interventions to manage these chronic conditions. Implantable devices such as pacemakers, defibrillators, and bio-resorbable stents offer effective solutions for regulating heart function and improving patient quality of life. This growing demand for implantable devices is a major market driver, as healthcare systems worldwide focus on providing efficient, long-term treatments for heart-related disorders.
- Minimally Invasive Procedures
The increasing preference for minimally invasive surgeries is driving the adoption of smaller, more efficient cardiac implant devices, such as stents and pacemakers. Patients and healthcare providers alike are opting for procedures that involve smaller incisions, shorter recovery times, and reduced risk of complications. These benefits have led to the development of advanced implantable devices that can be delivered through minimally invasive techniques. As a result, the demand for devices such as bio-resorbable stents and compact pacemakers has surged. This trend is a key market driver, as both patients and practitioners seek less invasive, yet effective, solutions for managing cardiovascular conditions.
Opportunities
- Integration of Smart Technologies
The integration of remote monitoring, data analytics, and wireless connectivity into cardiac bio implant devices presents a significant growth opportunity in the market. These innovations enable continuous, real-time tracking of heart health, providing valuable insights into patient conditions and facilitating personalized treatment plans. By leveraging data analytics, healthcare providers can detect early signs of complications or device malfunctions, allowing for timely interventions. This improves patient outcomes and enhances the efficiency of healthcare systems. The growing adoption of connected healthcare solutions is expected to drive the demand for smart cardiac implants, positioning this technology as a key market opportunity.
- Regenerative Medicine and Stem Cell Therapy
The integration of regenerative medicine techniques, particularly stem cell-based therapies, into cardiac bio implant devices offers a transformative market opportunity. By combining stem cell therapy with traditional implants, these devices have the potential to regenerate damaged heart tissue, improving long-term outcomes and reducing the need for repeated interventions. Stem cell-based implants could also offer more sustainable solutions for treating heart disease by promoting natural healing processes within the heart. As research and development in regenerative medicine continue to progress, the adoption of these advanced treatments is expected to expand, opening new avenues for innovation and growth in the cardiac implant market.
Restraints/Challenges
- Limited Awareness in Emerging Market
Despite the increasing demand for cardiac bio implant devices, awareness and adoption in many emerging markets remain limited, presenting a significant challenge to market growth. In these regions, factors such as lower healthcare budgets, lack of access to advanced medical technologies, and insufficient education on the benefits of implantable devices contribute to slower adoption rates. Furthermore, cultural factors and the availability of alternative treatments may hinder patients from opting for bio implants. This lack of awareness and infrastructure, coupled with limited healthcare resources, restricts the potential for widespread use of cardiac bio implants in emerging markets, slowing overall market expansion.
- High Cost of Devices
The high production costs associated with the advanced technologies and materials used in cardiac bio implant devices present a significant market restraint. These devices require cutting-edge components such as bio-compatible materials, smart technologies, and precision manufacturing processes, which all contribute to their high cost. As a result, the price of these implants can be prohibitive for both healthcare providers and patients, limiting their adoption, especially in emerging markets where healthcare budgets are often constrained. This cost barrier restricts access to life-saving treatments in these regions, hindering the widespread use of cardiac implants and limiting overall market growth.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Cardiac Bio Implant Devices Market Scope
The market is segmented on the basis of product, disease, and procedure. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Structural Cardiac Implants
- Stents and Related Implants
- A Pacing Device
Disease
- Myocardial Ischemia
- Acute Myocardial Infarction
- Arrhythmias
- Congestive Heart Failure (CHF)
- Angina Pectoris (AP)
- Cardiomyopathy
- Aortic Aneurism
- Others
Procedure
- Angioplasty
- Heart Valve Repair or Replacement
- Open Heart Surgery
- Minimally Invasive Heart Surgery
- Cardiac Resynchronization Therapy (CRT)
- Others
Cardiac Bio Implant Devices Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product, disease, and procedure as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America leads the cardiac bio implant devices market due to a significant aging population and a high prevalence of lifestyle-related diseases. The increasing number of elderly individuals and the rising incidence of heart conditions drive the demand for implantable cardiac devices. As a result, the region experiences strong market growth and adoption of advanced cardiac technologies.
Asia-Pacific is projected to experience significant growth in the cardiac bio implant devices market from 2025 to 2032, driven by a large patient population. The region is benefiting from increased government investment in healthcare and rising healthcare spending. These factors, combined with growing demand for advanced medical treatments, contribute to the market's expansion.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Cardiac Bio Implant Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Cardiac Bio Implant Devices Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Edwards Lifesciences Corporation (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- LivaNova PLC (U.K.)
- Medtronic (U.S.)
- Boston Scientific Corporation (U.S.)
- MicroPort Scientific Corporation (China)
- Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
- ClearPoint Neuro, Inc. (U.S.)
- Imricor (U.S.)
- Biotronik (Germany)
- ELESTIM-CARDIO (Russia)
- QualiMed (Germany)
- MEDICO S.R.L. (Italy)
- Lepu Medical Technology (Beijing) Co., Ltd. (China)
- W. L. Gore & Associates, Inc. (U.S.)
- LifeNet Health (U.S.)
Latest Developments in Cardiac Bio Implant Devices Market
- In December 2023, Roche signed a USD 2.7 billion cash agreement to acquire the U.S.-based Carmot Therapeutics. This acquisition strengthens Roche's position in the biotechnology sector. The deal is expected to enhance Roche's drug discovery capabilities, particularly in the areas of oncology and immunology
- In February 2021, Medtronic launched the TYRX Absorbable Antibacterial Envelope, designed to support implanted neurostimulators and cardiac implantable electrical devices. This one-time use envelope offers antimicrobial properties to reduce infection risks. It aims to enhance the safety and longevity of these medical implants by providing an additional layer of protection
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.