Global Blood Plasma Market
Market Size in USD Billion
CAGR :
% 
 USD
34.96 Billion
USD
78.27 Billion
2024
2032
USD
34.96 Billion
USD
78.27 Billion
2024
2032
| 2025 –2032 | |
| USD 34.96 Billion | |
| USD 78.27 Billion | |
|
|
|
|
Blood Plasma Market Size
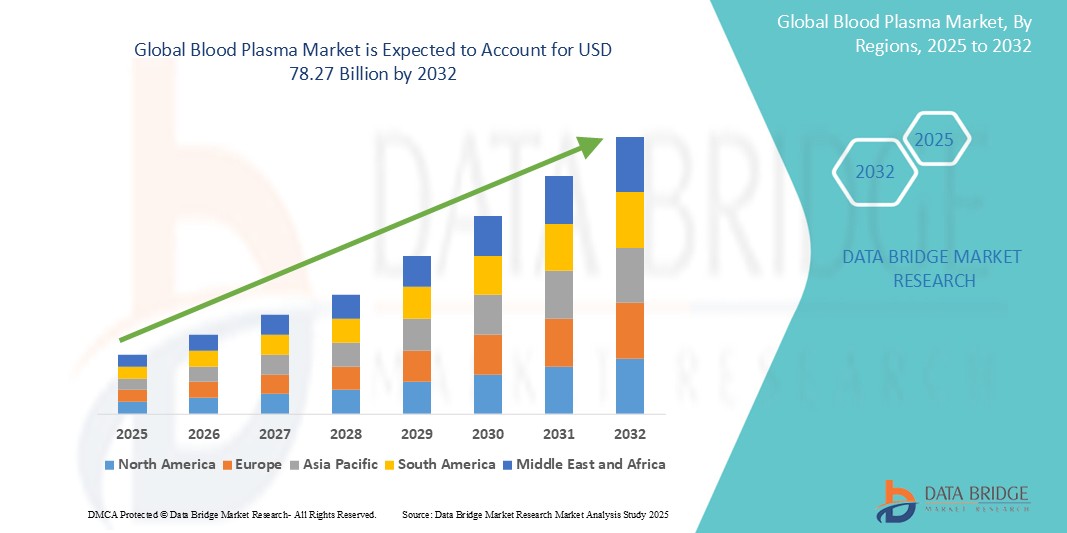
- The global blood plasma market size was valued at USD 34.96 billion in 2024 and is expected to reach USD 78.27 billion by 2032, at a CAGR of 10.60% during the forecast period
- The market growth is largely fueled by the rising demand for plasma-derived therapies for chronic and rare diseases, along with advancements in plasma collection and fractionation technologies
- Technological advancements in plasma collection, fractionation, and purification processes are significantly enhancing the efficiency and safety of plasma-derived therapies. Innovations such as improved donor screening, automated collection systems, and enhanced fractionation techniques are expanding plasma supply capacity while maintaining high product quality, further supporting market growth
- Expanding healthcare infrastructure in emerging economies, along with favorable government policies and reimbursement frameworks, is improving plasma collection networks and treatment accessibility. In addition growing investment by pharmaceutical companies and contract manufacturing organizations (CMOs) in plasma processing facilities is enabling scalable production to meet increasing global demand
Blood Plasma Market Analysis
- The global blood plasma market is largely shaped by the expanding use of plasma-derived therapies, which are crucial for treating a variety of chronic and rare medical conditions. For instance, therapies such as immunoglobulins and clotting factors have become essential in managing diseases such as immune deficiencies and bleeding disorders
- Increasing awareness among healthcare professionals and patients about the benefits and applications of plasma-derived treatments is influencing the market dynamics significantly. Educational campaigns and clinical research have enhanced understanding of how these therapies can improve quality of life for individuals with complex conditions
- North America dominates the blood plasma market with share of 47.05% in 2024, driven by a well-established healthcare infrastructure and high adoption of advanced plasma therapies
- Asia-pacific is expected to be the fastest growing region in the blood plasma market during the forecast period due to rising healthcare expenditures, growing awareness of plasma therapies, and expanding patient pools across emerging economies are key drivers
- The immunoglobulin segment dominates the largest market share with 45.09% in 2024, due to its widespread application in treating immune deficiencies and autoimmune diseases
Report Scope and Blood Plasma Market Segmentation
|
Attributes |
Blood Plasma Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Blood Plasma Market Trends
“Advancement in Plasma Therapies Boosting Market Growth”
- The blood plasma market is increasingly focused on the development of innovative plasma-derived therapies to address a wider range of chronic and rare diseases
- Advances in fractionation technology have improved the efficiency and purity of plasma products, enabling more effective treatments
- The growing number of plasma collection centers supports a more stable supply chain, ensuring consistent availability of plasma for therapeutic use
- Healthcare providers are placing greater emphasis on personalized treatment plans using plasma therapies to improve patient outcomes
- Immunoglobulin therapies are being tailored to specific patient needs, enhancing their effectiveness in managing immune-related disorders
- In conclusion, overall, the market is shifting towards more specialized and advanced plasma products that cater to evolving healthcare demands and improve quality of care
Blood Plasma Market Dynamics
Driver
“Rising Demand for Immunoglobulin and Plasma-Derived Therapies”
- The global rise in chronic and rare diseases such as primary immunodeficiency, hemophilia, and autoimmune disorders has significantly increased the demand for immunoglobulin and plasma-derived therapies
- The growing elderly population and improved access to healthcare services are contributing to higher treatment rates, thereby fueling plasma consumption in both developed and emerging markets
- Advancements in plasma fractionation now allow multiple therapeutic proteins to be extracted from a single donation, enhancing overall product yield and efficiency across production pipelines
- For instance, the U.S. and Germany have scaled outpatient use of plasma therapies, which supports better adherence and long-term patient management while easing pressure on inpatient facilities
- In conclusion, as global healthcare systems recognize the critical value of these therapies, investments in collection infrastructure and technological innovation by biopharmaceutical companies continue to rise, supporting market expansion and sustainability
Restraint/Challenge
“High Cost and Complex Manufacturing Process”
- The production of plasma-derived therapies is costly and highly complex, requiring extensive quality control, sophisticated infrastructure, and skilled personnel, which raises the overall cost of manufacturing
- Plasma must be collected under strict regulatory conditions with consistent donor screening, followed by multi-step processing including fractionation, purification, and pathogen inactivation that further complicate operations
- These procedures demand large investments in cold chain logistics and specialized equipment, which create significant barriers for new entrants and limit cost-effective expansion in low-income regions
- Setting up a single plasma donation center involves millions in capital expenditure, strict compliance protocols, and trained staff, making it less viable in developing healthcare systems
- In conclusion, these operational constraints result in limited global accessibility and higher prices for patients, reducing the pace of market growth and slowing adoption in resource-constrained areas where such therapies are urgently needed
Blood Plasma Market Scope
The market is segmented on the basis of type, communication protocol, unlocking mechanism, and application.
- By Type
On the basis of type, the blood plasma market is segmented into hyperimmune globulin, albumin, factor VIII, factor IX, immunoglobulin, and others. The immunoglobulin segment dominates the wth largest market share with 45.09% in 2024, due to its widespread application in treating immune deficiencies and autoimmune diseases. Its established clinical relevance, supported by increasing demand in chronic disease management, ensures its stronghold in the market.
The hyperimmune globulin segment is anticipated to witness the fastest growth from 2025 to 2032, driven by its use in targeted treatment of specific infectious diseases and its rising adoption in epidemic response strategies.
- By Mode of Delivery
On the basis of mode of delivery, the blood plasma market is segmented into infusion solutions, gels, sprays, and biomedical sealants. The infusion solutions segment holds the largest revenue share in 2024, attributed to their routine use in hospital settings for delivering plasma-derived therapies. Their clinical efficiency, safety profile, and physician preference contribute to their continued dominance.
The biomedical sealants segment anticipated to register the fastest growth, as these are increasingly utilized in surgeries for wound management and hemostasis, supported by innovation in bio-compatible materials.
- By Therapeutic Indication
On the basis of therapeutic indication, the blood plasma market is segmented into immunology, oncology, pulmonology, rheumatology, transplantation, neurology, hematology and other. The immunology segment leads the market in terms of revenue share in 2024, fueled by the high prevalence of immune-related disorders and the critical role plasma therapies play in their treatment.
The neurology segment is expected to register the fastest growth, as plasma-derived products are gaining recognition for their effectiveness in treating neurological conditions such as guillain-barré syndrome and chronic inflammatory demyelinating polyneuropathy.
- By Application
On the basis of application, the blood plasma market is segmented into hypogammaglobulinemia, immunodeficiency diseases, hemophilia, von willebrand's disease (vwd) and others. The immunodeficiency diseases segment dominates the market in 2024, due to the increasing global awareness and diagnosis of primary and secondary immunodeficiencies. Its reliance on long-term immunoglobulin therapy supports sustained growth.
The hemophilia segment is poised to witness the fastest growth, driven by advancements in factor replacement therapies and increased availability of recombinant plasma products.
- By End User
On the basis of end user, the blood plasma market is segmented into hospitals, clinics and other. The hospitals segment accounts for the largest revenue share in 2024, due to their extensive infrastructure for transfusion services, diagnostics, and emergency care. Hospitals also remain central to patient access and long-term therapy management.
The clinics segment is projected to register the fastest growth, supported by the decentralization of healthcare and rising demand for outpatient plasma therapy services, particularly for chronic disease management.
Blood Plasma Market Regional Analysis
- North America dominates the blood plasma market with the largest revenue share of 47.05% in 2024, driven by a well-established healthcare infrastructure and high adoption of advanced plasma therapies
- The region benefits from robust plasma collection networks and regulatory support, ensuring consistent supply and innovation in therapeutic development
- The presence of leading industry players and specialized research institutions contributes to ongoing clinical advancements and widespread availability of plasma-derived products
U.S. Blood Plasma Market Insight
The U.S. blood plasma market encompasses a wide range of applications, including immunology, neurology, hematology, and critical care. It is driven by rising demand for plasma-derived therapies to treat rare and chronic diseases such as primary immunodeficiencies, hemophilia, and autoimmune disorders. The market scope is further broadened by a well-established healthcare infrastructure, a growing donor base, and advanced collection and fractionation technologies. Leading pharmaceutical companies and contract manufacturing organizations (CMOs) are investing in expanding their plasma processing capacities, contributing to market growth.
Europe Blood Plasma Market Insight
The European blood plasma market covers applications such as immune deficiency treatments, coagulation disorders, and critical care. The scope is supported by favorable reimbursement policies, stringent regulatory standards, and increasing prevalence of chronic conditions. Efforts to boost plasma collection within the EU and cross-border collaborations among countries enhance supply stability. The presence of key market players and growing investments in research and development also strengthen the market’s scope across western and eastern Europe.
U.K. Blood Plasma Market Insight
In the U.K., the blood plasma market scope includes therapeutic areas such as immunology, neurology, and hematology, with a particular focus on treating rare and orphan diseases. The national health service (NHS) plays a crucial role in managing plasma-derived product distribution and ensuring access to essential treatments. An increase in awareness campaigns and regulatory support for donor recruitment is expanding the domestic plasma supply, thereby contributing to the availability and affordability of therapies.
Germany Blood Plasma Market Insight
Germany's blood plasma market spans across various medical specialties, with a strong emphasis on immunoglobulins, albumin, and clotting factors. The country’s rigorous regulatory environment and high healthcare standards ensure consistent quality and safety of plasma-derived therapies. With one of the largest healthcare systems in Europe, Germany’s scope includes both public and private healthcare providers, and it continues to invest in local plasma collection centers and biopharmaceutical innovations.
Asia-Pacific Blood Plasma Market Insight
The Asia-pacific blood plasma market features a broad scope across therapeutic applications such as immunology, infectious diseases, and emergency care. Rising healthcare expenditures, growing awareness of plasma therapies, and expanding patient pools across emerging economies are key drivers. Countries such as China, India, and Japan are investing in domestic plasma collection infrastructure and local manufacturing, while regional collaborations aim to address the rising demand for plasma-derived products.
Japan Blood Plasma Market Insight
Japan’s blood plasma market is defined by its focus on high-precision plasma fractionation and treatment of chronic and rare diseases, including immune deficiencies and neurological disorders. The market scope is enhanced by strong governmental backing for R&D, stringent quality controls, and a sophisticated healthcare delivery system. Additionally, Japan’s aging population and rising chronic disease burden necessitate a growing need for plasma-derived therapies.
China Blood Plasma Market Insight
China’s blood plasma market encompasses applications in immunoglobulin therapy, albumin supplementation, and treatment for hemophilia and other coagulation disorders. The country’s scope is reinforced by a rapidly developing healthcare infrastructure, large patient population, and government support for expanding domestic plasma collection networks. As local manufacturers increase production capabilities and regulatory frameworks evolve, the availability and scope of plasma therapies continue to grow rapidly.
Blood Plasma Market Share
The blood plasma industry is primarily led by well-established companies, including:
- Allergan (Ireland)
- AbbVie Inc. (U.S.)
- GALDERMA (Switzerland)
- Evolus, Inc. (U.S.)
- Revance (U.S.)
- HUGEL, Inc. (South Korea)
- Ipsen Pharma (France)
- USWM, LLC. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (UK)
- Merz Pharma (Germany)
- Medytox (South Korea)
- Smith+Nephew (U.S.)
- Sanofi (France)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- LGM Pharma. (U.S.)
- Lannett (U.S.)
- NorthStar Rx LLC (U.S.)
Latest Developments in Global Blood Plasma Market
- In January 2024, CSL Behring, a division of the renowned biotechnology company CSL, made a 10g prefilled syringe for Hizentra® (Immune Globulin Subcutaneous [Human] 20% Liquid) accessible to the public. The Hizentra prefilled syringes enhance the treatment experience for individuals with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) and Primary Immunodeficiency (PI) by eliminating the requirement to extract medication from vials
- In October 2023, KTC Edibles, the leading provider of edible oils in the U.K., introduced Planet Palm, a fresh line of palm oil products that are certified sustainable, traceable, and sustainably produced. These products are specifically designed for food producers in the U.K.
- In September 2023, Grifols announced the expansion of its plasma collection network in North America, aiming to increase access to high-quality plasma for immunotherapy and other treatments
- In August 2023, CSL Behring launched a new high-purity immunoglobulin product, enhancing treatment options for patients with immune deficiencies and autoimmune diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL BLOOD PLASMA MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL BLOOD PLASMA MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL BLOOD PLASMA MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR BLOOD PLASMA MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR BLOOD PLASMA MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR BLOOD PLASMA MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR BLOOD PLASMA MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR BLOOD PLASMA MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
18 GLOBAL BLOOD PLASMA MARKET, BY PRODUCT
18.1 OVERVIEW
18.2 IMMUNOGLOBULINS
18.2.1 BY TYPE
18.2.1.1. INTRAVENOUS IMMUNOGLOBULINS
18.2.1.2. SUBCUTANEOUS IMMUNOGLOBULINS
18.2.1.3. OTHER IMMUNOGLOBULINS
18.2.2 BY APPLICATION
18.2.2.1. NEUROLOGY
18.2.2.1.1. MYASTHENIA GRAVIS
18.2.2.1.2. GUILLAIN-BARRÉ SYNDROME
18.2.2.1.3. CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
18.2.2.1.4. OTHERS
18.2.2.2. IMMUNOLOGY
18.2.2.3. LUPUS
18.2.2.4. RHEUMATOID ARTHRITIS
18.2.2.5. SCLERODERMA
18.2.2.6. SJÖGREN'S SYNDROME
18.2.2.7. HEMATOLOGY
18.2.2.8. CRITICAL CARE
18.2.2.9. PULMONOLOGY
18.2.2.10. HEMATO-ONCOLOGY
18.2.2.11. RHEUMATOLOGY
18.2.2.12. OTHER APPLICATIONS
18.3 COAGULATION FACTOR CONCENTRATES
18.3.1 BT TYPE
18.3.1.1. FACTOR VIII
18.3.1.2. FACTOR IX
18.3.1.3. VON WILLEBRAND FACTOR
18.3.1.4. PROTHROMBIN COMPLEX CONCENTRATE
18.3.1.5. FIBRINOGEN CONCENTRATES
18.3.1.6. FACTOR XIII
18.3.2 BY APPLICATION
18.3.2.1. NEUROLOGY
18.3.2.1.1. MYASTHENIA GRAVIS
18.3.2.1.2. GUILLAIN-BARRÉ SYNDROME
18.3.2.1.3. CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
18.3.2.1.4. OTHERS
18.3.2.2. IMMUNOLOGY
18.3.2.3. LUPUS
18.3.2.4. RHEUMATOID ARTHRITIS
18.3.2.5. SCLERODERMA
18.3.2.6. SJÖGREN'S SYNDROME
18.3.2.7. HEMATOLOGY
18.3.2.8. CRITICAL CARE
18.3.2.9. PULMONOLOGY
18.3.2.10. HEMATO-ONCOLOGY
18.3.2.11. RHEUMATOLOGY
18.3.2.12. OTHER APPLICATIONS
18.4 ALBUMIN
18.4.1 BY TYPE
18.4.1.1. HUMAN SERUM ALBUMIN
18.4.1.2. RECOMBINANT ALBUMIN
18.4.1.3. IV ALBUMIN
18.4.2 BY APPLICATION
18.4.2.1. NEUROLOGY
18.4.2.1.1. MYASTHENIA GRAVIS
18.4.2.1.2. GUILLAIN-BARRÉ SYNDROME
18.4.2.1.3. CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
18.4.2.1.4. OTHERS
18.4.2.2. IMMUNOLOGY
18.4.2.3. LUPUS
18.4.2.4. RHEUMATOID ARTHRITIS
18.4.2.5. SCLERODERMA
18.4.2.6. SJÖGREN'S SYNDROME
18.4.2.7. HEMATOLOGY
18.4.2.8. CRITICAL CARE
18.4.2.9. PULMONOLOGY
18.4.2.10. HEMATO-ONCOLOGY
18.4.2.11. RHEUMATOLOGY
18.4.2.12. OTHER APPLICATIONS
18.5 HYPERIMMUNE GLOBINS
18.5.1 BY TYPE
18.5.1.1. HEPATITIS B IMMUNE GLOBULIN (HBIG)
18.5.1.2. CYTOMEGALOVIRUS IMMUNE GLOBULIN
18.5.1.3. VARICELLA-ZOSTER IMMUNE GLOBULIN
18.5.1.4. RHO(D) IMMUNE GLOBULIN
18.5.2 BY APPLICATION
18.5.2.1. NEUROLOGY
18.5.2.1.1. MYASTHENIA GRAVIS
18.5.2.1.2. GUILLAIN-BARRÉ SYNDROME
18.5.2.1.3. CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
18.5.2.1.4. OTHERS
18.5.2.2. IMMUNOLOGY
18.5.2.3. LUPUS
18.5.2.4. RHEUMATOID ARTHRITIS
18.5.2.5. SCLERODERMA
18.5.2.6. SJÖGREN'S SYNDROME
18.5.2.7. HEMATOLOGY
18.5.2.8. CRITICAL CARE
18.5.2.9. PULMONOLOGY
18.5.2.10. HEMATO-ONCOLOGY
18.5.2.11. RHEUMATOLOGY
18.5.2.12. OTHER APPLICATIONS
18.6 PROTEASE INHIBITORS
18.6.1 BY TYPE
18.6.1.1. ALPHA-1 ANTITRYPSIN (AAT)
18.6.1.2. C1 ESTERASE INHIBITOR (C1-INH)
18.6.1.3. ANTITHROMBIN III
18.6.2 BY APPLICATION
18.6.2.1. NEUROLOGY
18.6.2.1.1. MYASTHENIA GRAVIS
18.6.2.1.2. GUILLAIN-BARRÉ SYNDROME
18.6.2.1.3. CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
18.6.2.1.4. OTHERS
18.6.2.2. IMMUNOLOGY
18.6.2.3. LUPUS
18.6.2.4. RHEUMATOID ARTHRITIS
18.6.2.5. SCLERODERMA
18.6.2.6. SJÖGREN'S SYNDROME
18.6.2.7. HEMATOLOGY
18.6.2.8. CRITICAL CARE
18.6.2.9. PULMONOLOGY
18.6.2.10. HEMATO-ONCOLOGY
18.6.2.11. RHEUMATOLOGY
18.6.2.12. OTHER APPLICATIONS
18.7 OTHER PRODUCTS
19 GLOBAL BLOOD PLASMA MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 NEUROLOGY
19.2.1 MYASTHENIA GRAVIS
19.2.2 GUILLAIN-BARRÉ SYNDROME
19.2.3 CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY
19.2.4 OTHERS
19.3 IMMUNOLOGY
19.4 LUPUS
19.5 RHEUMATOID ARTHRITIS
19.6 SCLERODERMA
19.7 SJÖGREN'S SYNDROME
19.8 HEMATOLOGY
19.9 CRITICAL CARE
19.1 PULMONOLOGY
19.11 HEMATO-ONCOLOGY
19.12 RHEUMATOLOGY
19.13 OTHER APPLICATIONS
20 GLOBAL BLOOD PLASMA MARKET, BY PROCESSING TECHNOLOGY
20.1 OVERVIEW
20.2 ION-EXCHANGE CHROMATOGRAPHY
20.3 AFIINITY CHROMATOGRAPHY
20.4 CRYOPRECIPITATION
20.5 ULTRAFILTRATION
20.6 MICROFILTRATION
21 GLOBAL BLOOD PLASMA MARKET, BY MODE
21.1 MODERN PLASMA FRACTIONATION
21.2 TRADITIONAL PLASMA FRACTIONATION
22 GLOBAL BLOOD PLASMA MARKET, BY AGE GROUP
22.1 OVERVIEW
22.2 PEDIATRIC
22.3 ADULT
22.4 GERIATRIC
23 GLOBAL BLOOD PLASMA MARKET, BY END USER
23.1 OVERVIEW
23.2 HOSPITALS & CLINICS
23.3 CLINICAL RESEARCH LABORATORIES
23.4 ACADEMIC INSTITUTES
23.5 OTHERS
24 GLOBAL BLOOD PLASMA MARKET, BY DISTRIBUTION CHANNEL
24.1 OVERVIEW
24.2 DIRECT TENDERS
24.3 THIRD PARTY DISTRIBUTION
24.4 OTHERS
25 GLOBAL BLOOD PLASMA MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL BLOOD PLASMA MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL BLOOD PLASMA MARKET, COMPANY PROFILE
27.1 BIOTEST AG
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPEMENTS
27.2 CSL
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPEMENTS
27.3 GC BIOPHARMA CORP
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPEMENTS
27.4 GRIFOILS
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPEMENTS
27.5 INTAS PHARMACEUTICALS LTD.
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPEMENTS
27.6 KEDRION S.P.A.
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPEMENTS
27.7 LFB
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPEMENTS
27.8 OCTAPHARMA AG
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPEMENTS
27.9 SANQUIN
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPEMENTS
27.1 TAKEDA PHARMACEUTICALS
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPEMENTS
27.11 ABBVIE, INC.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPEMENTS
27.12 IPSEN PHARMA
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPEMENTS
27.13 SUN PHARMACEUTICALS INDUSTRIES, LTD.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPEMENTS
27.14 PFIZER, INC.
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPEMENTS
27.15 EUROPLASMA
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPEMENTS
27.16 IMMUNOTEK BIO CENTERS
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPEMENTS
27.17 KAMADA PHARMACEUTICALS
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPEMENTS
27.18 PROMETIC PLASMA RESOURCES
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPEMENTS
27.19 PLASMA INDUCTION (INDIA) PVT LTD.
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPEMENTS
27.2 HAEMONETICS CORPORATION
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPEMENTS
27.21 CHINA BIOLOGIC PRODUCTS
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.22 KM BIOLOGICS
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.23 SHANXI KANGBAO BIOLOGICAL PRODUCTS CO., LTD
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.24 SICHUAN YUANDA SHUYANG PHARMACEUTICAL CO., LTD
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.25 ADMA BIOLOGICS, INC.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPEMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.