Global Biomanufacturing Viral Detection and Quantification Market, By Offering Type (Consumables, Instruments, Services), Technology (PCR, ELISA, Flow Cytometry, Plaque Assay, Others), Application (Blood and Blood Products Manufacturing, Vaccines and Therapeutics Manufacturing, Cellular and Gene Therapy Products Manufacturing, Stem Cell Products Manufacturing, Tissue and Tissue Products Manufacturing), End User (Life Science Companies, Testing Laboratories, CROs, CDMOs) – Industry Trends and Forecast to 2031.
Biomanufacturing Viral Detection and Quantification Market Analysis and Size
The Global Biomanufacturing Viral Detection and Quantification Market is witnessing robust growth driven by increasing demand for biopharmaceuticals, stringent regulatory requirements, and advancements in detection technologies. Key trends include the adoption of novel detection methods such as CRISPR-based diagnostics, the integration of artificial intelligence, and the shift towards single-use technologies.
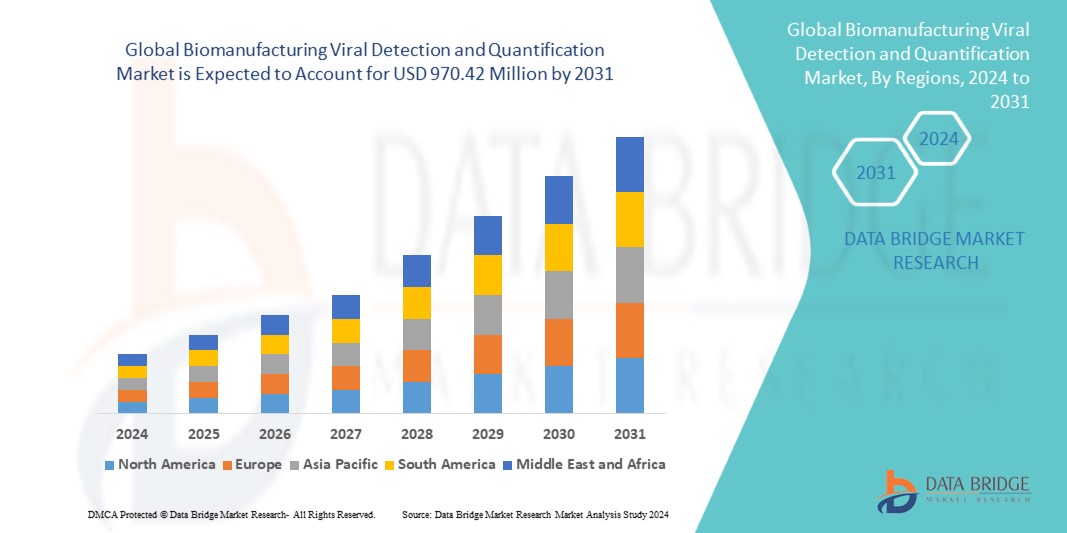
Data Bridge Market Research analyses that the global biomanufacturing viral detection and quantification market which was USD 474.00 million in 2023, and expected to reach upto USD 970.42 million by 2031, and is expected to undergo a CAGR of 9.37% during the forecast period. This indicates that the market value. “PCR” dominates the technology segment of the global biomanufacturing viral detection and quantification market due to its effectiveness in identifying and measuring viral nucleic acids during biopharmaceutical production. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016-2021)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Offering Type (Consumables, Instruments, Services), Technology (PCR, ELISA, Flow Cytometry, Plaque Assay, Others), Application (Blood and Blood Products Manufacturing, Vaccines and Therapeutics Manufacturing, Cellular and Gene Therapy Products Manufacturing, Stem Cell Products Manufacturing, Tissue and Tissue Products Manufacturing), End User (Life Science Companies, Testing Laboratories, CROs, CDMOs)
|
|
Countries Covered
|
U.S., Canada, Mexico, U.K., Germany, France, Spain, Italy, Netherlands, Switzerland, Russia, Belgium, Turkey, Rest of Europe, China, South Korea, Japan, India, Australia, Singapore, Malaysia, Indonesia, Thailand, Philippines, Rest of Asia-Pacific, South Africa, Rest of Middle East and Africa, Brazil, Rest of South America
|
|
Market Players Covered
|
Thermo Fisher Scientific Inc. (U.S.), Merck KGaA (Germany), Sartorius AG (Germany), Charles River Laboratories (U.S.), Danaher Corporation (U.S.), Bio-Rad Laboratories, Inc. (U.S.), QIAGEN (Netherlands), Lonza Group Ltd. (Switzerland), Pall Corporation (U.S.), PerkinElmer, Inc. (U.S.), GE Healthcare (U.S.), Roche Diagnostics International Ltd. (Switzerland), BD (U.S.), Promega Corporation (U.S.), FUJIFILM Diosynth Biotechnologies (U.S.), Avantor, Inc. (U.S.), SGS Societe Generale de Surveillance SA. (Switzerland), Eurofins Scientific (Luxembourg), Virapur LLC (U.S.), BioReliance Corporation (U.S.)
|
|
Market Opportunities
|
|
Market Definition
The global biomanufacturing viral detection and quantification market encompasses the products, services, and technologies utilized for detecting and quantifying viral contaminants in biopharmaceutical manufacturing processes. It includes a wide range of methods such as polymerase chain reaction (PCR), immunoassays, sequencing, and other molecular techniques tailored to identify and quantify viruses during various stages of biopharmaceutical production, including cell line development, upstream and downstream processing, and final product testing.
Global Biomanufacturing Viral Detection and Quantification Market Dynamics
Drivers
- Increasing Demand for Biopharmaceuticals
The growing demand for biopharmaceutical products, including vaccines, monoclonal antibodies, and recombinant proteins, is driving the need for stringent viral detection and quantification measures throughout the biomanufacturing process to ensure product safety and quality.
- Technological Advancements in Technologies
Ongoing advancements in detection technologies, including PCR-based assays, next-generation sequencing, and novel immunoassays, are enhancing the sensitivity, specificity, and speed of viral detection and quantification methods, driving market growth and adoption.
- Emerging Infectious Diseases and Pandemic Preparedness
Outbreaks of infectious diseases such as COVID-19 highlight the importance of rapid and accurate viral detection in biomanufacturing. The need for pandemic preparedness and response measures is fueling investment in advanced viral detection and quantification technologies, bolstering market growth.
- Growing Investment for Healthcare Facilities
Surging focus towards improving the condition of healthcare facilities and improving the overall healthcare infrastructure another important factor fostering the growth of the market. Rising number of partnerships and strategic collaborations between the public and private players pertaining to funding and application of new and improved technology is further creating lucrative market opportunities.
Opportunities
- Adoption of Point-of-Care Testing (POCT)
The shift towards decentralized testing and point-of-care diagnostics presents opportunities for the development of rapid, portable, and user-friendly viral detection devices. POCT platforms for viral detection and quantification offer benefits such as real-time monitoring, reduced turnaround times, and increased accessibility, creating new avenues for market growth, particularly in resource-limited settings and remote locations.
- Integration of Artificial Intelligence (AI) and Machine Learning (ML)
The integration of AI and ML technologies into viral detection and quantification processes enables the analysis of large datasets, predictive modeling, and pattern recognition, enhancing the accuracy and efficiency of detection methods. Leveraging AI/ML algorithms for data analysis and interpretation presents opportunities for developing innovative viral detection solutions with improved sensitivity, specificity, and speed, driving market growth and differentiation.
Restraints
- High Cost of Advanced Technologies
The adoption of advanced viral detection and quantification technologies often involves significant upfront capital investment, and ongoing maintenance and operational costs. The high cost of equipment, reagents, and skilled personnel required for these technologies can act as a barrier to entry for smaller biomanufacturing companies, limiting market growth and adoption.
- Regulatory Complexity and Compliance Challenges
Compliance with stringent regulatory requirements for viral safety in biopharmaceutical manufacturing is essential but can be complex and time-consuming. Navigating the regulatory landscape, obtaining approvals, and ensuring ongoing compliance with evolving guidelines pose challenges for market players, particularly smaller companies with limited resources and expertise, slowing down market growth and innovation.
Challenges
- Technical Limitations of Detection Methods
Despite advancements in viral detection technologies, certain limitations persist, such as the inability to detect all viral contaminants or differentiate between active and inactive viruses. Variability in sensitivity, specificity, and reliability among detection methods can also pose challenges, impacting the accuracy and reliability of results and hindering market adoption.
- Resistance to Change and Technological Adoption
The biomanufacturing industry, characterized by long development timelines and stringent quality requirements, can be slow to adopt new technologies and processes. Resistance to change, reliance on established methods, and concerns about disrupting production workflows can impede the adoption of innovative viral detection and quantification technologies, constraining market growth and innovation in the short term.
This global biomanufacturing viral detection and quantification market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global biomanufacturing viral detection and quantification market contact Data Bridge Market Research for an analyst brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- No recent development related to the market.
Global Biomanufacturing Viral Detection and Quantification Market Scope
The global biomanufacturing viral detection and quantification market is segmented on the basis of offering type, technology, application, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Offering Type
- Consumables
- Instruments
- Services
Technology
- PCR
- ELISA
- Flow Cytometry
- Plaque Assay
- Others
Application
- Blood and Blood Products Manufacturing
- Vaccines and Therapeutics Manufacturing
- Cellular and Gene Therapy Products Manufacturing
- Stem Cell Products Manufacturing
- Tissue and Tissue Products Manufacturing
End user
- Life Science Companies
- Testing Laboratories
- CROs
- CDMOs
Global Biomanufacturing Viral Detection and Quantification Market Regional Analysis/Insights
The global biomanufacturing viral detection and quantification market is analyzed and market size insights and trends are provided by country offering type, technology, application, and end user as referenced above.
The countries covered in the global biomanufacturing viral detection and quantification market report are U.S., Canada, Mexico, U.K., Germany, France, Spain, Italy, Netherlands, Switzerland, Russia, Belgium, Turkey, Rest of Europe, China, Japan, India, Australia, South Korea, Singapore, Thailand, Malaysia, Indonesia, Philippines, rest of Asia-Pacific, Brazil, rest of South America in South America, South Africa, and rest of Middle East and Africa in Middle East and Africa.
North America region dominate the global biomanufacturing viral detection and quantification market due to the rise of large PCR test products manufacturers, the presence of major market players and increased technological advancement in the region.
Asia-Pacific region is projected to observe significant amount of growth in the global biomanufacturing viral detection and quantification market due to the rapid necessity for telemedicine and healthcare support. Moreover, the modernization and development in telecommunication is further anticipated to propel the growth of the global biomanufacturing viral detection and quantification market in the region in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The global biomanufacturing viral detection and quantification market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for global biomanufacturing viral detection and quantification market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the global biomanufacturing viral detection and quantification market. The data is available for historic period 2016-2021.
Competitive Landscape and Global Biomanufacturing Viral Detection and Quantification Market Share Analysis
The global biomanufacturing viral detection and quantification market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global biomanufacturing viral detection and quantification market.
Some of the major players operating in the global biomanufacturing viral detection and quantification market are:
- Thermo Fisher Scientific Inc. (U.S.)
- Merck KGaA (Germany)
- Sartorius AG (Germany)
- Charles River Laboratories (U.S.)
- Danaher Corporation (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- QIAGEN (Netherlands)
- Lonza Group Ltd. (Switzerland)
- Pall Corporation (U.S.)
- PerkinElmer, Inc. (U.S.)
- GE Healthcare (U.S.)
- Roche Diagnostics International Ltd. (Switzerland)
- BD (U.S.), Promega Corporation (U.S.)
- FUJIFILM Diosynth Biotechnologies (U.S.)
- Avantor, Inc. (U.S.)
- SGS Societe Generale de Surveillance SA. (Switzerland)
- Eurofins Scientific (Luxembourg)
- Virapur LLC (U.S.)
- BioReliance Corporation (U.S.)
SKU-