Global Biological Safety Testing Products And Services Market
Market Size in USD Billion
CAGR :
% 
 USD
3.69 Billion
USD
9.26 Billion
2021
2029
USD
3.69 Billion
USD
9.26 Billion
2021
2029
| 2022 –2029 | |
| USD 3.69 Billion | |
| USD 9.26 Billion | |
|
|
|
|
Biological Safety Testing Products and Services Market Analysis and Size
Biological safety testing services are used to confirm the absence of bacterial contaminants in vaccines and biopharmaceuticals. Toxicology testing, bioburden testing, and analytical testing are services provided by companies that evaluate products based on parameters such as accuracy, specificity, linearity, and range. A biological product is a substance derived from a living organism that is used in disease prevention or treatment. Antitoxins, bacterial and viral vaccines, blood products, and hormone extracts are instances of these products. Biotechnology can produce these products in a living system, such as a microorganism or plant cell.
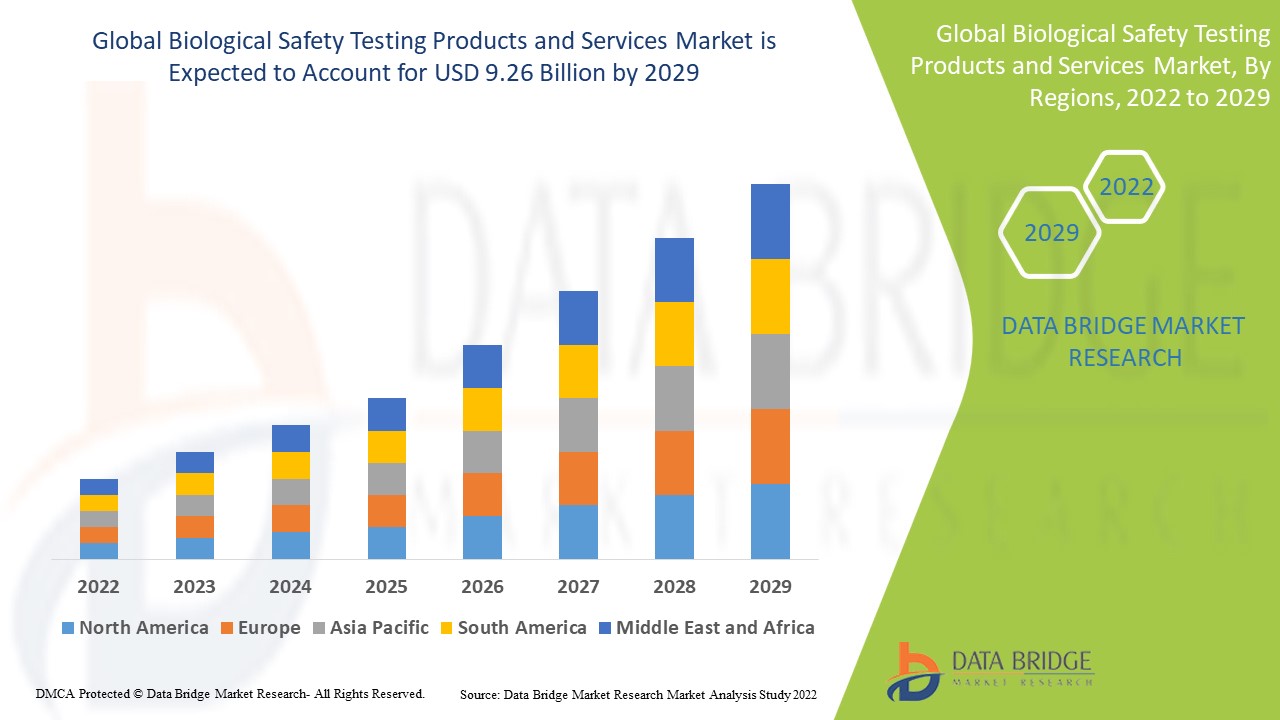
Data Bridge Market Research analyses that the biological safety testing products and services market which was USD 3.69 billion in 2021, is expected to reach USD 9.26 billion by 2029, at a CAGR of 12.2% during the forecast period 2022 to 2029. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Biological Safety Testing Products and Services Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Kits and Reagents, Instruments and Services), Test (Endotoxin Test, Sterility Test, Residual Host Contamination Detection Tests, Cell Line Authentication and Characterization Tests, Bioburden Tests, Adventitious Agent Detection Test and Others), Application (Blood and Blood-Based Products, Gene Therapy, Tissue and Tissue-based Products, Vaccines and Therapeutics, Stem Cells and Others), End-Use (Pharmaceuticals and Biotechnology Companies, Contract Development and Manufacturing Companies, Research and Academia and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Pace Analytical Services LLC (U.S.), Creative Biogene (U.S.), ViruSure GmbH. (Germany), Toyobo Co, Ltd (Japan), Samsung Biologics (South Korea), Sartorius AG (Germany), Charles River Laboratories (U.S.), BSL Bioservice (Germany), Lonza (Switzerland), BioOutsource Ltd. (U.S.), Samsung BioLogics (South Korea), Thermo Fisher Scientific Inc. (U.S.), Merck KGaA (Germany), SGS Société Générale de Surveillance SA (Switzerland), WuXi AppTec (China), Eurofins Scientific (Luxembourg) |
|
Market Opportunities |
|
Market Definition
Biological safety testing is performed to ensure that biopharmaceutical products such as drugs, therapeutics, vaccines, blood and blood-derived products, stem cell, tissue banks, and other biologics are free of contamination. The biological safety testing industry includes biologics controlled manufacturing products and services. The products category is further subdivided into kits and regents and instruments. Biological safety testing is a process to ensure the products and services are free from contamination and to decontaminate biological medicines, laboratories, and healthcare institutions. Biological safety and product testing is the strength for the growth of biopharmaceutical and vaccines, biological safety testing products and innovative organic pharmaceutical development.
Global Biological Safety Testing Products and Services Market Dynamics
Drivers
- Rising drug approvals by the U.S. food and drug administration (USFDA)
The USFDA's increasing number of drug approvals has accelerated market growth. In 2018, the Center for Drug Evaluation and Research (CDER) approved more than 50 new drugs and biologics. Balversa (Erdafitinib) tablet was approved by the FDA in 2019 for the treatment of metastatic bladder cancer. The FDA has also approved the 'Therascreen FGFR RGQ-PCR Kit,' which is used for therapeutic purposes. Aside from that, the growing emphasis of pharmaceutical manufacturing companies on avoiding transmission and maximising drug safety drives the use of biological safety testing instruments.
- Numerous vaccines in developmental phase
Blood and blood-based products, vaccines and therapeutics, stem cells, and other products are all subject to biological safety testing. Biological safety testing for vaccines and therapeutics will grow rapidly in the coming years. Growing disease burdens, as well as increased research and development for new vaccines and therapies, will have a significant impact on segmental growth. According to the Association of the British Pharmaceutical Industry, more than 250 vaccines are in development for the treatment and prevention of various diseases. Furthermore, several research institutes are providing low-cost third-party services to maintain product quality and make drug development procedures more efficient and painless, thereby increasing the use of biological safety testing products and services market.
Opportunities
- Rising prevalence of lifestyle-related chronic diseases
The rising prevalence of lifestyle-related chronic diseases such as cancer and diabetes is a major factor driving the increase in drug development and subsequent commercialization. This is expected to increase the need for scrutinising and ensuring the safety of targeted and specialised therapies, as well as evaluating outcomes and avoiding potential flaws. Furthermore, pharmaceutical and biotechnology firms are increasingly incorporating biological safety testing products and services in order to produce highly potent and contamination-free biologics in order to meet the large number of people suffering from target diseases. Viral safety testing is proving to be one of the most pressing concerns for most businesses, as it is a critical component of biologics chemistry, manufacturing, and control. This is creating market growth opportunities.
Restraints/Challenges
- Stringent regulations
Stringent regulations and complicated procedure to obtain FDA approval will obstruct the market's growth rate. The risk of viral contamination poses a significant risk to human use of biological products.
This biological safety testing products and services market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the biological safety testing products and services market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Biological Safety Testing Products and Services Market
The COVID-19 had a devastating impact on the world, with the death toll rising at an alarming rate every day. According to the Centers for Disease Control and Prevention (CDC), new laboratory tests for identifying COVID-19 specimens have been introduced, accelerating market growth. The state and local public health departments are working hard to collect the specimens, which is driving up demand for biological safety testing products and services. The World Health Organization (WHO) has suggested that new point-of-care immunodiagnostic tests be used in research settings. Due to the National Public Health Emergency, the Indian Council of Medical Research (ICMR) enabled subsidised tests. To enable test operations, market participants are supplying diagnostic test kits to private labs. The ICMR instructed the laboratories to undertake appropriate biosafety and biosecurity precautions while collecting samples. For biological safety testing, ICMR has urged the laboratories and hospitals to create a separate disease-specific sample collection site.
Recent developments
- In 2021, Charles River Laboratories, Inc. (US) launched a new detection tool named EndoScan-V, validated endotoxin detection and measurement software used to produce and report numerical test data. The software performed the obligatory measurements and calculations and generated test reports with the convenience of digital signature report approval.
Global Biological Safety Testing Products and Services Market Scope
The biological safety testing products and services market is segmented on the basis of product, application, test type and end-use. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Reagents and kits
- Instruments
- Services
Application
- Vaccines and Therapeutics
- Blood and Blood-based Products
- Gene Therapy
- Tissue and Tissue-based Products
- Stem Cell
Test Type
- Endotoxin Tests
- Sterility Tests
- Cell Line Authentication and Characterization Tests
- Bioburden Tests
- Adventitious Agent Detection Tests
- Residual Host Contamination Detection Tests
- Others
End-Use
- Pharmaceuticals and Biotechnology Companies
- Contract Development and Manufacturing Companies
- Research and Academia
- Others
Biological Safety Testing Products and Services Market Regional Analysis/Insights
The biological safety testing products and services market is analysed and market size insights and trends are provided by country, product, application, test type and end-use as referenced above.
The countries covered in the biological safety testing products and services market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the biological safety testing products and services market due to increased adoption in cancer research, and the development of new biologics, vaccines and drugs. Moreover, an increasing number of R&D investments by companies is one of the factors responsible for growth.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 owing to increasing awareness about the benefits of these products and rising healthcare spending are expected to contribute to growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The biological safety testing products and services market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for biological safety testing products and services market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the biological safety testing products and services market. The data is available for historic period 2010-2020.
Competitive Landscape and Biological Safety Testing Products and Services Market Share Analysis
The biological safety testing products and services market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to biological safety testing products and services market.
Some of the major players operating in the biological safety testing products and services market are:
- Sartorius AG (Germany)
- Charles River Laboratories (U.S.)
- BSL Bioservice (Germany)
- Lonza (Switzerland)
- Milliporesigma
- BioOutsource Ltd. (U.S.)
- Samsung BioLogics (South Korea)
- Thermo Fisher Scientific Inc. (U.S.)
- Merck KGaA (Germany)
- SGS Société Générale de Surveillance SA (Switzerland)
- WuXi AppTec (China)
- Eurofins Scientific (Luxembourg)
- Pace Analytical Services LLC (U.S.)
- Creative Biogene (U.S.)
- ViruSure GmbH. (Germany)
- Toyobo Co, Ltd (Japan)
- Samsung Biologics (South Korea)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













