Global At Home Testing Kits Market
Market Size in USD Billion
CAGR :
% 
 USD
7.89 Billion
USD
12.67 Billion
2024
2032
USD
7.89 Billion
USD
12.67 Billion
2024
2032
| 2025 –2032 | |
| USD 7.89 Billion | |
| USD 12.67 Billion | |
|
|
|
|
At-Home Testing Kits Market Size
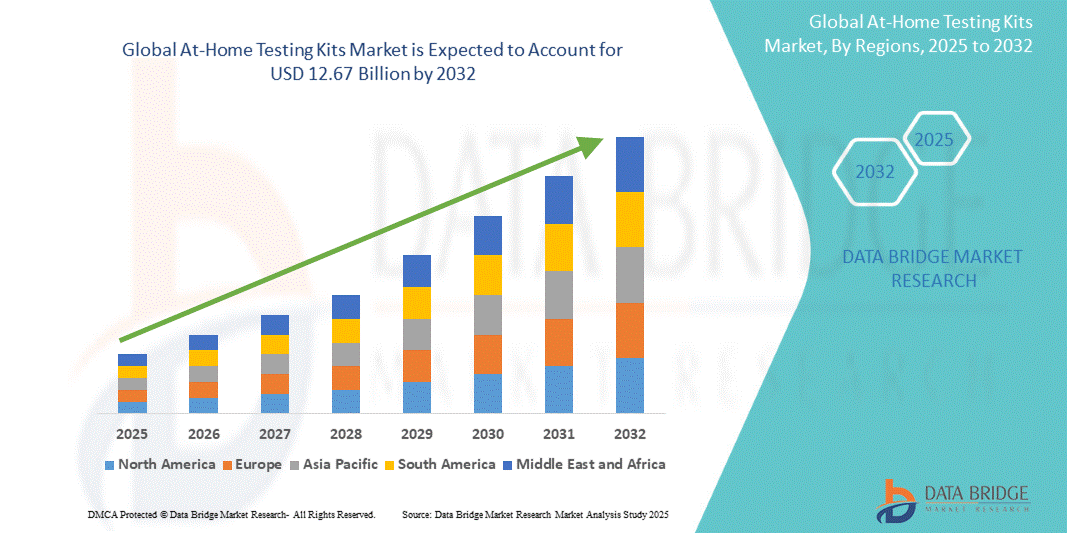
- The global At-home testing kits market size was valued at USD 7.89 billion 2024 and is expected to reach USD 12.67 billion by 2032, at a CAGR of 6.1% during the forecast period
- The market growth is largely driven by increasing consumer demand for convenient, private, and rapid health diagnostics, especially post-pandemic, which has heightened health awareness and decentralized healthcare access
- Furthermore, advancements in diagnostic technologies, greater availability of direct-to-consumer lab services, and rising prevalence of chronic and infectious diseases are contributing to the widespread adoption of home-based test kits. These converging factors are accelerating the demand for self-testing solutions, thereby significantly propelling the industry's expansion
At-Home Testing Kits Market Analysis
- At-home testing kits, enabling individuals to perform diagnostic tests for various health conditions, are becoming crucial components of consumer-driven healthcare due to their convenience, affordability, and ability to deliver rapid results without clinical intervention
- The escalating demand for At-home testing kits is primarily fueled by rising health awareness, a growing preference for private and remote healthcare solutions, and the increasing burden of chronic and infectious diseases requiring regular monitoring
- North America dominated the At-home testing kits market with the largest revenue share of 39.5% in 2024, supported by advanced healthcare infrastructure, the proliferation of telehealth platforms, and a strong presence of key diagnostics companies, with the U.S. leading the adoption due to increased consumer confidence in self-testing, especially in chronic condition management and women’s health
- Asia-Pacific is expected to be the fastest growing region in the At-home testing kits market during the forecast period due to expanding healthcare access, digital literacy, and rising consumer spending on preventive care
- Glucose test segment dominated the At-home testing kits market with a market share of 35% in 2024, driven by the global surge in diabetes prevalence and the widespread use of self-monitoring blood glucose devices for effective disease management
Report Scope and At-Home Testing Kits Market Segmentation
|
Attributes |
At-Home Testing Kits Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
At-Home Testing Kits Market Trends
“Shift Toward Personalized, Digital, and Preventive Healthcare”
- A significant and accelerating trend in the global at-home testing kits market is the growing integration of digital health platforms and personalized diagnostic technologies, allowing individuals to monitor, diagnose, and manage health conditions remotely with increased accuracy and privacy
- For instance, companies such as LetsGetChecked and Everlywell provide personalized health reports through app-based platforms that integrate test results with virtual consultations and treatment guidance, enabling users to take proactive control of their health from home
- The adoption of AI-driven analytics and mobile health apps is transforming traditional testing kits into comprehensive health solutions. These platforms track historical data, offer automated health insights, and notify users of abnormal trends. For instance, myLAB Box includes digital dashboards that help users understand test results and follow recommended next steps
- Furthermore, the growing acceptance of telehealth services is driving demand for test kits that link seamlessly with remote care providers. Users can now test for conditions such as STDs, food sensitivities, or hormone levels and share results in real time with healthcare professionals
- The emphasis on early detection and prevention, combined with rising consumer interest in self-care and wellness, is encouraging companies such as Cue Health and Healthtracka to expand their offerings with broader test panels and real-time data access through cloud-connected diagnostic tools
- This trend toward digitized, personalized, and preventive home diagnostics is reshaping consumer expectations in healthcare and accelerating the expansion of the at-home testing kits market across both developed and emerging regions
At-Home Testing Kits Market Dynamics
Driver
“Consumer Shift Toward Convenience and Preventive Health Monitoring”
- The increasing demand for convenient, private, and accessible diagnostic solutions is a major driver for the at-home testing kits market, especially as consumers take a more proactive role in managing their health
- For instance, in February 2024, Everlywell expanded its women's health portfolio with a new comprehensive hormone testing kit that offers at-home sample collection, lab testing, and detailed insights via its digital platform—making personalized hormone tracking more accessible than ever
- The COVID-19 pandemic further normalized home diagnostics, boosting consumer confidence and regulatory support for at-home testing solutions, which now span a wide array of health conditions beyond infectious diseases
- In addition, at-home testing kits offer unique advantages such as discretion, reduced waiting times, and the ability to monitor chronic conditions such as diabetes or high cholesterol without visiting a clinic
- The convenience of direct delivery, easy sample collection, and digital result access is attracting both tech-savvy and medically underserved populations. This is further supported by the increasing availability of subscription-based services that provide routine test kits for ongoing health monitoring
Restraint/Challenge
“Accuracy Concerns and Regulatory Hurdles”
- While at-home testing kits offer ease and accessibility, concerns over test accuracy, improper sample collection, and result misinterpretation remain significant challenges, potentially limiting adoption among skeptical consumers and healthcare providers
- For instance, studies have shown variability in sensitivity and specificity across different brands and test types, especially for complex conditions such as food sensitivities or hormonal imbalances, leading to potential misdiagnoses or delayed treatments
- In addition, the need for stringent regulatory approval from bodies such as the FDA, CE, and other national health authorities adds complexity and slows the rollout of new diagnostic kits, particularly in markets with evolving compliance frameworks
- Misuse or misunderstanding of test results, especially in the absence of professional medical interpretation, can further impact consumer trust. To mitigate this, companies such as LetsGetChecked and Cue Health have started incorporating optional physician consultations to accompany test results
- Moreover, disparities in digital literacy and healthcare access in low-income or rural regions may hinder the widespread adoption of these kits, despite their potential benefits. Overcoming these barriers through improved user education, transparent performance data, and affordable pricing models will be critical to driving broader acceptance and sustainable growth in the market
At-Home Testing Kits Market Scope
The market is segmented on the basis of test type, type, age group, sample type, usage, and distribution channel.
- By Test Type
On the basis of test type, the at-home testing kits market is segmented into pregnancy test, HIV test kit, diabetes, infectious diseases, glucose tests, ovulation predictor test kit, drug abuse test kit, and other test types. The glucose tests segment dominated the market with the largest market revenue share of 35% in 2024, driven by the increasing global prevalence of diabetes and the growing importance of routine blood sugar monitoring. Consumers with diabetes rely heavily on at-home glucose tests to manage their condition effectively, encouraging high-volume usage and steady market demand. The accessibility and affordability of glucose test kits further bolster their widespread adoption, especially in emerging economies.
The infectious diseases segment is anticipated to witness the fastest growth rate of 19.2% from 2025 to 2032, fueled by increased health awareness, the aftereffects of the COVID-19 pandemic, and rising demand for rapid diagnostic kits for conditions such as influenza, HIV, and STDs. The segment is also being driven by product innovation and the increasing availability of combination panels that can test for multiple infections simultaneously.
- By Type
On the basis of type, the at-home testing kits market is segmented into cassette, strip, midstream, test panel, dip card, and other form types. The strip segment dominated the market with the largest market revenue share of 36.4% in 2024, due to its widespread use in glucose and pregnancy testing. Strip-based tests are valued for their low cost, ease of use, and quick results, making them ideal for large-scale home-based testing across all income groups.
The midstream segment is anticipated to witness the fastest growth rate of 17.8% from 2025 to 2032, driven by its growing popularity in pregnancy and ovulation tests. Midstream kits offer enhanced hygiene, ease of handling, and improved accuracy, making them a preferred choice among consumers looking for user-friendly solutions.
- By Age
On n the basis of age, the at-home testing kits market is segmented into pediatric, adult, and geriatric. The adult segment dominated the market with the largest market revenue share of 61.3% in 2024, reflecting the broad consumer base using self-testing kits for fertility, chronic conditions, STDs, and lifestyle-related health monitoring. Adults increasingly prefer the privacy, convenience, and empowerment associated with home-based diagnostics, fueling sustained demand in this category.
The geriatric segment is anticipated to witness the fastest growth rate of 16.5% from 2025 to 2032, driven by rising cases of chronic illnesses such as cardiovascular diseases, diabetes, and cancer. The growing need for non-invasive, routine monitoring solutions among elderly populations is expected to accelerate segment growth.
- By Sample Type
On the basis of sample type, the at-home testing kits market is segmented into urine, blood, saliva, and others. The urine segment held the largest market revenue share of 42.1% in 2024, supported by its use across several high-demand test categories including pregnancy, ovulation, drug abuse, and urinary tract infections. Urine-based tests are highly preferred due to their non-invasiveness, ease of collection, and wide diagnostic scope.
The saliva segment is expected to witness the fastest growth rate of 18.4% from 2025 to 2032, attributed to advancements in non-invasive testing and rising demand for painless alternatives in infectious disease detection and genetic testing. Increasing consumer comfort and innovation in saliva-based diagnostics are driving this trend.
- By Usage
On the basis of usage, the at-home testing kits market is segmented into disposable and reusable. The disposable segment dominated the market with the largest market revenue share of 68.2% in 2024, due to high adoption in routine and single-use diagnostic tests such as pregnancy, ovulation, and glucose monitoring. Disposable kits are cost-effective, easy to use, and require no maintenance, making them ideal for one-time or low-frequency users.
The reusable segment is expected to grow at the fastest CAGR of 15.1% from 2025 to 2032, driven by increasing use of digital health monitoring devices, particularly in chronic condition management. Devices such as continuous glucose monitors (CGMs) and reusable lancets are gaining popularity among patients who require regular testing and long-term tracking.
- By Distribution Channel
On the basis of distribution channel, the at-home testing kits market is segmented into retail pharmacies, drug stores, supermarkets/hypermarkets, and online pharmacies. The online pharmacies segment dominated the market with the largest revenue share of 39.8% in 2024, supported by the rapid expansion of e-commerce platforms, discreet purchasing options, and growing consumer preference for home delivery of medical supplies.
The retail pharmacies segment is projected to witness steady growth of 11.6% CAGR from 2025 to 2032, particularly in emerging economies where pharmacies remain the most trusted and accessible point of care. In-person consultation and immediate availability of products continue to make retail channels vital for at-home test kit distribution.
At-Home Testing Kits Market Regional Analysis
- North America dominated the at-home testing kits market with the largest revenue share of 39.5% in 2024, supported by advanced healthcare infrastructure, the proliferation of telehealth platforms, and a strong presence of key diagnostics companies, with the U.S. leading the adoption due to increased consumer confidence in self-testing, especially in chronic condition management and women’s health
- Consumers in the region increasingly favor the convenience, privacy, and real-time access to results offered by at-home diagnostic kits across a wide range of health conditions, including fertility, chronic diseases, and infectious illnesses
- This growing acceptance is further supported by high healthcare spending, a well-developed regulatory framework, and the strong presence of leading diagnostics companies and telehealth platforms, positioning at-home testing kits as an essential part of modern healthcare delivery in both urban and remote areas
U.S. At-Home Testing Kits Market Insight
The U.S. at-home testing kits market captured the largest revenue share of 83.2% in 2024 within North America, driven by rising consumer awareness, widespread adoption of telehealth, and a strong preference for convenience and privacy in healthcare. The growing demand for self-testing in areas such as chronic disease management, reproductive health, and infectious diseases is accelerating market expansion. Moreover, the presence of major players, favorable FDA regulatory support, and increasing availability of digital platforms offering lab-certified results and virtual consultations are further strengthening market growth.
Europe At-Home Testing Kits Market Insight
The Europe at-home testing kits market is projected to expand at a substantial CAGR throughout the forecast period, supported by rising health consciousness, improved healthcare access, and increased investment in personalized diagnostics. The shift toward preventive care and early detection is encouraging the use of home-based diagnostics for conditions ranging from fertility and hormone imbalances to food sensitivities. In addition, strong e-pharmacy growth and favorable regulatory reforms are enabling broader market penetration across both urban and rural areas.
U.K. At-Home Testing Kits Market Insight
The U.K. at-home testing kits market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by growing demand for direct-to-consumer health solutions and the rising adoption of digital health technologies. Consumers are increasingly opting for discreet, remote diagnostics for STDs, allergies, and general wellness, driven by NHS support for telehealth integration and growing availability of subscription-based test services. The emphasis on early detection and convenience continues to push demand forward.
Germany At-Home Testing Kits Market Insight
The Germany at-home testing kits market is expected to expand at a considerable CAGR during the forecast period, supported by the country’s strong healthcare infrastructure and increasing focus on personalized medicine. Consumers in Germany are becoming more proactive in managing health through regular home testing for conditions such as diabetes, thyroid disorders, and infections. The preference for certified, lab-backed home kits and the integration with teleconsultation platforms are contributing to sustained growth in the German market.
Asia-Pacific At-Home Testing Kits Market Insight
The Asia-Pacific at-home testing kits market is poised to grow at the fastest CAGR of 23.1% during the forecast period of 2025 to 2032, driven by rising health awareness, rapid digitalization, and improved access to healthcare solutions in countries such as China, India, and Japan. The region's growing demand for affordable, easy-to-use health diagnostics is encouraging domestic innovation and adoption. Government support for telehealth, combined with increasing consumer spending on wellness, is accelerating market penetration across both urban and rural populations.
Japan At-Home Testing Kits Market Insight
The Japan at-home testing kits market is gaining momentum due to the nation’s tech-savvy population, high demand for preventive care, and preference for discreet diagnostic solutions. Home testing is increasingly used for lifestyle diseases, women’s health, and infectious conditions. Japan’s aging population is also fueling the need for simple, non-invasive testing solutions, with strong growth potential in chronic condition monitoring. Integration with health tracking apps and smart devices is further enhancing user experience and driving market demand.
India At-Home Testing Kits Market Insight
The India at-home testing kits market accounted for the largest revenue share in Asia Pacific in 2024, propelled by a rapidly expanding middle class, widespread smartphone usage, and strong government focus on digital healthcare. Rising consumer demand for affordable, accessible diagnostics across urban and semi-urban regions is boosting growth. Local manufacturers are increasingly offering home testing solutions for glucose, pregnancy, infections, and lifestyle diseases, making India a key market in the region’s self-testing landscape.
At-Home Testing Kits Market Share
The At-home testing kits industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Siemens Healthcare GmbH (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- BD (U.S.)
- Drägerwerk AG & Co. KGaA (Germany)
- LifeScan IP Holdings, LLC (U.S.)
- Ascensia Diabetes Care Holdings AG (Switzerland)
- ACON Laboratories, Inc. (U.S.)
- Quidel Corporation (U.S.)
- ARKRAY USA, Inc. (U.S.)
- BTNX INC. (Canada)
- Atomo Diagnostics (Australia)
- Eurofins Scientific (Luxembourg)
- Piramal Enterprises Ltd. (India)
- Bionime Corporation (Taiwan)
- Nova Biomedical (U.S.)
- Cardinal Health (U.S.)
- OraSure Technologies (U.S.)
- Biolytical Laboratories Inc. (Canada)
- Everlywell, Inc. (U.S.)
- SA Scientific Ltd. (U.S.)
What are the Recent Developments in Global At-Home Testing Kits Market?
- In April 2023, Everlywell, a prominent U.S.-based health testing company, expanded its test menu with the launch of new women's health panels, including perimenopause and polycystic ovary syndrome (PCOS) testing kits. These additions reflect the company’s commitment to offering comprehensive, accessible at-home diagnostics for underrepresented health conditions. The expansion underscores the growing consumer demand for personalized, convenient testing solutions and strengthens Everlywell’s position in the evolving at-home healthcare landscape
- In March 2023, LetsGetChecked, a global health insights company, announced a collaboration with Walgreens to offer at-home health test kits through retail stores and online platforms. This partnership aims to bridge the gap between traditional retail pharmacies and direct-to-consumer testing, improving accessibility to diagnostic services. The initiative marks a strategic move to broaden consumer reach while enhancing convenience for those seeking health monitoring without clinical visits
- In March 2023, Cue Health, a California-based diagnostics firm, introduced a new COVID-19 and influenza molecular test in a single at-home cartridge. The test delivers lab-quality results in about 25 minutes using Cue’s proprietary connected device and mobile app. This development illustrates the trend toward multipurpose diagnostics and the integration of advanced technology into home testing, supporting faster, data-driven health decisions
- In February 2023, myLAB Box expanded its STD testing line with new combination panels that test for up to 14 different infections from a single sample. This advancement addresses the rising demand for privacy, speed, and comprehensiveness in sexual health testing. The company’s continued innovation reinforces its role in advancing accessible and confidential diagnostic care across the U.S.
- In January 2023, Healthtracka, a Nigerian health tech startup, launched a mobile-based platform to facilitate the ordering, sample collection, and results delivery of at-home diagnostic tests. By integrating telehealth consultations with diagnostics, the company is addressing key healthcare gaps in emerging markets. The initiative highlights the growing importance of digital health in expanding access to reliable at-home testing, particularly in underserved regions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.