Global Aerospace And Life Sciences Tic Market
Market Size in USD Billion
CAGR :
% 
 USD
39.73 Billion
USD
51.92 Billion
2025
2033
USD
39.73 Billion
USD
51.92 Billion
2025
2033
| 2026 –2033 | |
| USD 39.73 Billion | |
| USD 51.92 Billion | |
|
|
|
|
Aerospace and Life Sciences TIC Market Size
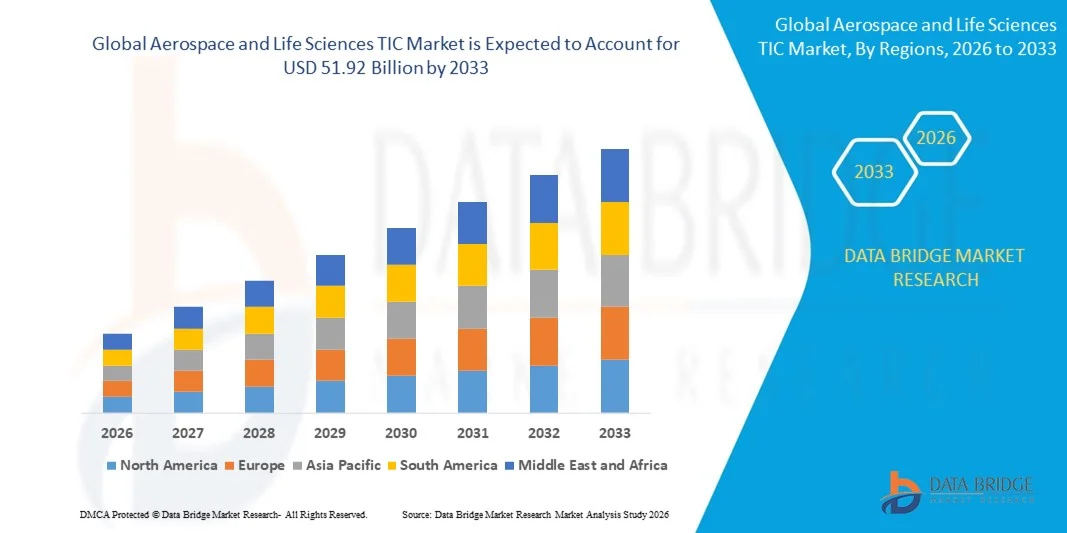
- The global aerospace and life sciences TIC market size was valued at USD 39.73 billion in 2025 and is expected to reach USD 51.92 billion by 2033, at a CAGR of 3.40% during the forecast period
- The market growth is largely fuelled by the increasing demand for quality, safety, and compliance services in aerospace and life sciences industries
- Rising adoption of advanced testing, inspection, and certification services to meet regulatory standards is driving market expansion
Aerospace and Life Sciences TIC Market Analysis
- The market is witnessing steady growth due to stringent regulatory requirements and the need for high-quality standards in products and processes
- Increasing globalization of aerospace and pharmaceutical supply chains is boosting demand for TIC services to ensure reliability, safety, and compliance
- North America dominated the aerospace and life sciences TIC market with the largest revenue share of 38.75% in 2025, driven by the presence of leading aerospace and medical device manufacturers, stringent regulatory requirements, and the high adoption of third-party testing and certification services
- Asia-Pacific region is expected to witness the highest growth rate in the global aerospace and life sciences TIC market, driven by rapid industrialization, expansion of aerospace and healthcare infrastructure, increasing regulatory oversight, and rising adoption of TIC services to ensure product safety and compliance
- The outsourced services segment held the largest market revenue share in 2025 driven by the growing preference for third-party experts to ensure regulatory compliance, operational efficiency, and cost-effectiveness. Outsourced TIC providers often offer specialized capabilities, advanced testing infrastructure, and global reach, making them a preferred choice for companies aiming to streamline quality assurance processes
Report Scope and Aerospace and Life Sciences TIC Market Segmentation
|
Attributes |
Aerospace and Life Sciences TIC Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand. |
Aerospace and Life Sciences TIC Market Trends
Rising Demand For Advanced Testing, Inspection, And Certification Services
- The growing emphasis on safety, quality, and regulatory compliance is significantly shaping the aerospace and life sciences TIC market, as companies increasingly require third-party verification and validation for products and processes. TIC services are gaining traction due to their ability to ensure product reliability, maintain industry standards, and mitigate operational risks. This trend strengthens their adoption across aerospace, pharmaceutical, and biotechnology sectors, encouraging service providers to innovate with new testing and inspection solutions
- Increasing awareness around stringent regulatory requirements, patient safety, and product traceability has accelerated the demand for TIC services in medical devices, pharmaceuticals, and aerospace components. Organizations are actively seeking comprehensive certification and testing solutions to comply with global standards, prompting TIC companies to expand service offerings and invest in advanced analytical capabilities
- Regulatory and compliance trends are influencing procurement decisions, with organizations emphasizing accreditation, quality assurance, and standardized testing. These factors are helping TIC providers differentiate services in a competitive market and build client trust, while also driving the adoption of ISO certifications, GMP compliance, and industry-specific standards. Companies are increasingly using marketing campaigns to highlight these capabilities to reinforce brand credibility and appeal to regulatory-conscious clients
- For instance, in 2024, SGS in Switzerland and Bureau Veritas in France expanded their aerospace and life sciences portfolios by incorporating advanced testing, inspection, and certification solutions. These launches were introduced in response to rising demand for rigorous quality assurance and compliance, with services offered across global industrial and laboratory networks. The offerings were also marketed as reliable, standardized solutions, enhancing client confidence and long-term engagement
- While demand for TIC services is growing, sustained market expansion depends on continuous technological upgrades, cost-effective operations, and maintaining accreditation across multiple geographies. Providers are also focusing on improving operational scalability, enhancing analytical capabilities, and developing innovative solutions that balance speed, accuracy, and compliance for broader adoption
Aerospace and Life Sciences TIC Market Dynamics
Driver
Growing Emphasis On Regulatory Compliance And Quality Assurance
- Increasing regulatory scrutiny and the need for rigorous quality standards is a major driver for the aerospace and life sciences TIC market. Organizations are increasingly relying on third-party testing, inspection, and certification to ensure safety, reduce risk, and maintain compliance with industry regulations. This trend is also pushing service providers to adopt advanced technologies, such as automated testing and digital verification tools, supporting service diversification
- Expanding applications in aerospace, medical devices, pharmaceuticals, and biotechnology are influencing market growth. TIC services help ensure operational reliability, safety, and standard compliance while maintaining industry-specific requirements, enabling organizations to meet regulatory expectations. The increasing complexity of products and manufacturing processes further reinforces this trend
- Service providers are actively promoting TIC-based solutions through innovation, digital platforms, and global accreditation. These efforts are supported by the growing industry demand for regulatory adherence and high-quality outcomes, and they also encourage partnerships between TIC companies and manufacturers to improve operational performance and reduce compliance risk
- For instance, in 2023, Intertek in the U.K. and TUV SUD in Germany reported increased adoption of TIC services in aerospace component validation and pharmaceutical quality assurance. This expansion followed higher industry demand for standardized testing, traceability, and safety compliance, driving repeat engagements and market differentiation. Both companies also highlighted digital reporting and accreditation standards in marketing campaigns to strengthen client trust and long-term contracts
- Although rising regulatory and quality trends support growth, wider adoption depends on cost optimization, service availability, and technology integration. Investment in digitalization, advanced analytical tools, and global laboratory networks will be critical for meeting industry demand and maintaining competitive advantage
Restraint/Challenge
High Cost And Stringent Regulatory Barriers
- The relatively high cost of TIC services compared to in-house testing remains a key challenge, limiting adoption among small and medium enterprises. Complex testing protocols, specialized equipment, and global accreditation requirements contribute to elevated pricing. In addition, varying international standards can further affect service accessibility and market penetration
- Awareness and understanding of TIC benefits remain uneven, particularly in emerging markets where regulatory frameworks are still developing. Limited knowledge of compliance requirements restricts adoption across certain industries. This also leads to slower uptake in regions where educational initiatives on TIC services are minimal
- Operational and logistical challenges also impact market growth, as TIC providers require certified laboratories, skilled personnel, and adherence to strict testing standards. Complex workflows, lengthy validation processes, and the need for specialized equipment increase operational costs. Companies must invest in laboratory infrastructure, training, and efficient logistics to maintain service integrity
- For instance, in 2024, aerospace and pharmaceutical clients in India and Brazil reported slower uptake of TIC services due to higher costs and limited awareness of international compliance requirements. Laboratory accreditation and inspection capacity were additional barriers. These factors also prompted some companies to rely on in-house testing, affecting demand for third-party TIC services
- Overcoming these challenges will require cost-efficient operations, expanded global presence, and targeted educational initiatives for manufacturers and regulatory bodies. Collaboration with industry associations, standardization organizations, and technology providers can help unlock the long-term growth potential of the global aerospace and life sciences TIC market. Furthermore, developing cost-effective service models and strengthening marketing strategies around compliance, accuracy, and efficiency will be essential for widespread adoption
Aerospace and Life Sciences TIC Market Scope
The market is segmented on the basis of sourcing type, service type, and application.
- By Sourcing Type
On the basis of sourcing type, the aerospace and life sciences TIC market is segmented into in-house and outsourced services. The outsourced services segment held the largest market revenue share in 2025 driven by the growing preference for third-party experts to ensure regulatory compliance, operational efficiency, and cost-effectiveness. Outsourced TIC providers often offer specialized capabilities, advanced testing infrastructure, and global reach, making them a preferred choice for companies aiming to streamline quality assurance processes.
The in-house segment is expected to witness the fastest growth rate from 2026 to 2033, driven by increasing investments in internal testing and inspection capabilities to maintain tighter control over critical processes. In-house TIC services are particularly valued for their flexibility, confidentiality, and immediate response, making them increasingly adopted by large aerospace and life sciences organizations for core quality assurance functions.
- By Service Type
On the basis of service type, the market is segmented into testing services, inspection services, certification services, and other services. The testing services segment held the largest revenue share in 2025, fueled by the rising demand for rigorous product validation and compliance with stringent safety and regulatory standards across aerospace and life sciences sectors. Testing services cover material, chemical, biological, and mechanical assessments, which are critical for ensuring product reliability and safety.
The certification services segment is expected to witness the fastest growth from 2026 to 2033, driven by the increasing regulatory complexity and the need for official compliance documentation. Certification services help organizations demonstrate adherence to international standards, reduce liability risks, and enhance market acceptance.
- By Application
On the basis of application, the market is segmented into medical and life sciences and aerospace. The medical and life sciences segment held the largest market revenue share in 2025 due to the high demand for rigorous testing, inspection, and certification of medical devices, pharmaceuticals, and biotech products to ensure patient safety and regulatory compliance.
The aerospace segment is expected to witness the fastest growth rate from 2026 to 2033, driven by the expansion of commercial and defense aviation, stringent safety regulations, and increasing adoption of advanced materials that require specialized TIC services.
Aerospace and Life Sciences TIC Market Regional Analysis
- North America dominated the aerospace and life sciences TIC market with the largest revenue share of 38.75% in 2025, driven by the presence of leading aerospace and medical device manufacturers, stringent regulatory requirements, and the high adoption of third-party testing and certification services
- Companies in the region highly value the expertise, advanced testing infrastructure, and compliance assurance offered by TIC providers, enabling faster product approvals and enhanced operational efficiency
- This widespread adoption is further supported by strong R&D investments, well-established supply chains, and a focus on quality and safety, establishing TIC services as a critical component for both aerospace and medical and life sciences sectors
U.S. Aerospace and Life Sciences TIC Market Insight
The U.S. aerospace and life sciences TIC market captured the largest revenue share in 2025 within North America, fueled by the increasing complexity of aerospace components and the demand for rigorous testing of medical devices and pharmaceuticals. Organizations are prioritizing compliance with FDA, ISO, and FAA standards, while the growing trend of outsourcing specialized TIC services is further driving market growth. Moreover, advancements in automation and digital testing solutions are enhancing efficiency and accuracy, significantly contributing to market expansion.
Europe Aerospace and Life Sciences TIC Market Insight
The Europe aerospace and life sciences TIC market is expected to witness the fastest growth rate from 2026 to 2033, primarily driven by stringent regulatory frameworks, such as EASA and MDR, and the increasing adoption of advanced aerospace and medical technologies. The region’s emphasis on safety, precision, and quality, combined with growing demand for outsourced TIC services, is fostering market growth. Companies in Europe are also investing in in-house TIC capabilities to maintain operational control and compliance across aerospace and medical applications.
U.K. Aerospace and Life Sciences TIC Market Insight
The U.K. aerospace and life sciences TIC market is expected to witness the fastest growth rate from 2026 to 2033, driven by the increasing demand for medical device testing, certification, and inspection services. The country’s focus on high-quality manufacturing and safety standards, along with the adoption of smart testing and inspection technologies, supports TIC service expansion. The U.K.’s robust healthcare and aerospace sectors further encourage companies to engage TIC providers for regulatory compliance and product validation.
Germany Aerospace and Life Sciences TIC Market Insight
The Germany aerospace and life sciences TIC market is expected to witness the fastest growth rate from 2026 to 2033, fueled by the country’s strong emphasis on innovation, precision engineering, and regulatory compliance. Germany’s well-established aerospace and medical industries drive the adoption of both in-house and outsourced TIC services. Integration of digital testing, automated inspection solutions, and advanced certification processes is becoming increasingly prevalent, meeting the growing demand for safe and high-quality products.
Asia-Pacific Aerospace and Life Sciences TIC Market Insight
The Asia-Pacific aerospace and life sciences TIC market is expected to witness the fastest growth rate from 2026 to 2033, driven by rapid industrialization, rising investments in aerospace and healthcare infrastructure, and increasing regulatory oversight in countries such as China, Japan, and India. The region’s growing manufacturing capabilities, coupled with a rising focus on compliance and quality assurance, are supporting the expansion of TIC services. In addition, government initiatives promoting safety standards and export quality further boost market adoption.
Japan Aerospace and Life Sciences TIC Market Insight
The Japan aerospace and life sciences TIC market is expected to witness the fastest growth rate from 2026 to 2033 due to the country’s focus on high-tech manufacturing, stringent regulatory requirements, and demand for quality assurance in medical devices and aerospace components. Japanese companies increasingly rely on TIC providers for testing, inspection, and certification services, integrating these solutions with advanced manufacturing and digital compliance systems.
China Aerospace and Life Sciences TIC Market Insight
The China aerospace and life sciences TIC market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to the country’s rapidly growing aerospace and medical device industries, increasing exports, and strict quality standards. The demand for third-party TIC services is rising as domestic manufacturers seek compliance with international regulations. Investments in advanced testing laboratories, skilled personnel, and automation further propel the market in China.
Aerospace and Life Sciences TIC Market Share
The Aerospace and Life Sciences TIC industry is primarily led by well-established companies, including:
- Intertek Group plc (U.K.)
- SGS SA (Switzerland)
- Bureau Veritas (France)
- MISTRAS (U.S.)
- TÜV NORD GROUP (Germany)
- TÜV SUD (Germany)
- Eurofins Scientific (Luxembourg)
- TÜV Rheinland (Germany)
- Applus+ (Spain)
- Element Material Technology (U.K.)
- DNV GL (Norway)
- UL LLC (U.S.)
- ALS Limited (Australia)
- Medistri SA (Belgium)
- Avomeen Analytical Services (U.S.)
- Gateway Analytical (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Global Aerospace And Life Sciences Tic Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Aerospace And Life Sciences Tic Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Aerospace And Life Sciences Tic Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.