Global Acute Ischemic Stroke Diagnosis And Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.79 Billion
USD
3.01 Billion
2024
2032
USD
1.79 Billion
USD
3.01 Billion
2024
2032
| 2025 –2032 | |
| USD 1.79 Billion | |
| USD 3.01 Billion | |
|
|
|
|
Acute Ischemic Stroke Diagnosis and Treatment Market Size
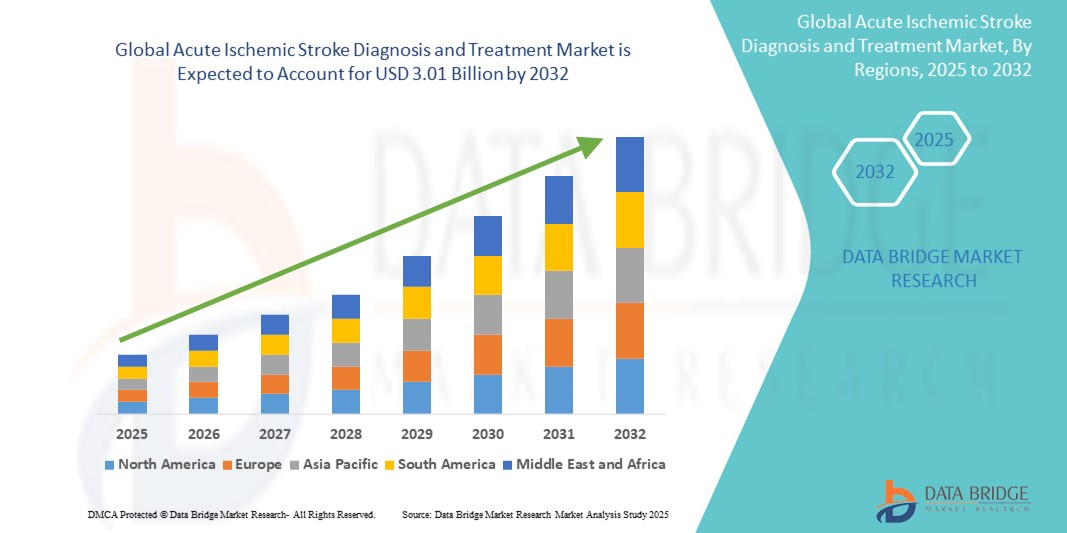
- The global acute ischemic stroke diagnosis and treatment market size was valued at USD 1.79 billion in 2024 and is expected to reach USD 3.01 billion by 2032, at a CAGR of 6.70% during the forecast period
- The market growth is largely fueled by the growing adoption and technological advancements in diagnostic imaging modalities, telemedicine, and neurointerventional procedures, leading to enhanced stroke identification and faster clinical decision-making across both hospital and ambulatory care settings
- Furthermore, rising awareness about the critical need for early diagnosis and treatment of stroke, coupled with increased investment in healthcare infrastructure and stroke care programs, is positioning advanced neuroimaging systems and thrombolytic therapies as essential components of stroke management. These converging factors are accelerating the uptake of acute ischemic stroke diagnosis and treatment solutions, thereby significantly boosting the industry's growth
Acute Ischemic Stroke Diagnosis and Treatment Market Analysis
- Acute ischemic stroke (AIS) is a critical medical condition that requires prompt and accurate diagnosis followed by immediate treatment to reduce long-term disability and mortality. Advanced imaging techniques and rapid therapeutic interventions have become vital components in managing AIS effectively
- The rising prevalence of stroke, increasing geriatric population, and heightened awareness about early diagnosis and treatment options are primary factors driving growth in the AIS diagnosis and treatment market globally
- North America dominated the acute ischemic stroke diagnosis and treatment market with the largest revenue share of 38.7% in 2024, attributed to a well-established healthcare infrastructure, high adoption of advanced diagnostic technologies such as CT/MRI, and a strong presence of pharmaceutical and medtech companies. The U.S., in particular, has seen a surge in mechanical thrombectomy procedures and increased access to primary stroke centers, propelling market expansion
- Asia-Pacific is expected to be the fastest growing region in the acute ischemic stroke diagnosis and treatment market with CAGR of 7.7% during the forecast period, driven by rising stroke incidence, improving healthcare access, growing medical tourism, and increasing government investments in healthcare infrastructure in countries such as China, India, and Japan
- Endovascular mechanical thrombectomy segment dominated the acute ischemic stroke diagnosis and treatment market with a market share of 47.2% in 2024, due to its high success rate in treating large vessel occlusions and its expanding adoption in comprehensive stroke centers. The procedure’s ability to rapidly restore blood flow in eligible patients has made it a critical component in acute stroke intervention protocols
Report Scope and Acute Ischemic Stroke Diagnosis and Treatment Market Segmentation
|
Attributes |
Acute Ischemic Stroke Diagnosis and Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Acute Ischemic Stroke Diagnosis and Treatment Market Trends
“Enhanced Convenience Through AI and Voice Integration”
- A significant and accelerating trend in the global acute ischemic stroke diagnosis and Treatment market is the deepening integration of artificial intelligence (AI) into imaging technologies and clinical decision support systems. This is significantly enhancing the speed and accuracy of diagnosis while aiding in timely treatment decisions
- For instance, AI-powered platforms such as Viz.ai and RapidAI use machine learning algorithms to analyze CT and MRI scans, detecting large vessel occlusions in real time and automatically alerting stroke teams. This enables faster triage and significantly improves door-to-needle times for thrombolysis and thrombectomy procedures
- AI-assisted tools can learn from patient data to predict outcomes, recommend personalized treatment pathways, and support neurologists in prioritizing cases, especially in high-volume emergency department
- Moreover, AI integration within stroke networks and telehealth systems enables rapid image sharing and virtual consultation, particularly valuable in rural and underserved regions with limited access to stroke specialists
- This trend toward more intelligent, automated, and responsive stroke care systems is fundamentally reshaping treatment protocols and improving patient outcomes. Consequently, companies such as Siemens Healthineers, GE HealthCare, and Philips are actively developing and marketing AI-integrated imaging and diagnostic solutions tailored for acute ischemic stroke
Acute Ischemic Stroke Diagnosis and Treatment Market Dynamics
Driver
“Growing Need Due to Rising Stroke Incidence and Advancements in Diagnostic & Therapeutic Technologies”
- The increasing global prevalence of acute ischemic strokes, driven by rising geriatric populations, sedentary lifestyles, and increasing incidences of cardiovascular diseases, is significantly propelling the demand for advanced diagnosis and treatment solutions
- For instance, in April 2024, Medtronic announced the expansion of its stroke care portfolio through the development of AI-powered neurovascular imaging technologies. Such strategic advancements are expected to drive the Acute Ischemic Stroke Diagnosis and Treatment market during the forecast period
- Growing awareness among patients and healthcare providers regarding early diagnosis and timely intervention is boosting the adoption of diagnostic tools such as MRI, CT, and cerebral angiography. These tools are essential for the prompt identification of stroke type and severity, enabling clinicians to initiate effective treatment within the golden hour
- Furthermore, government and private initiatives focused on improving stroke management infrastructure and funding neurological research are contributing to increased market growth. The introduction of public stroke awareness campaigns and telemedicine-enabled stroke diagnosis in rural and underserved areas are widening patient access to care
- The increasing adoption of minimally invasive procedures, such as mechanical thrombectomy and endovascular interventions, along with thrombolytic agents like tissue plasminogen activator (tPA), is transforming the stroke treatment paradigm and supporting market expansion
Restraint/Challenge
“High Cost of Advanced Imaging and Limited Access in Low-Resource Settings”
- The high cost associated with advanced imaging modalities such as MRI and CT, as well as mechanical thrombectomy devices, continues to limit market penetration, especially in developing economies. Hospitals and diagnostic centers in low- and middle-income countries often face budget constraints, leading to underutilization of advanced technologies
- In addition, limited healthcare infrastructure, shortage of trained neurologists, and lack of awareness about stroke symptoms in rural areas further restrict timely diagnosis and treatment
Reimbursement challenges also pose a significant hurdle for patients, as insurance coverage for acute stroke therapies varies across regions, and out-of-pocket expenses can be prohibitively high
- Moreover, while AI and telemedicine are expected to bridge some of these gaps, their implementation is still in early stages in many parts of the world due to digital illiteracy and inconsistent internet access
- To overcome these challenges, key stakeholders must focus on cost reduction through innovation, government support for stroke care funding, expansion of public health programs, and education on the importance of early stroke intervention
Acute Ischemic Stroke Diagnosis and Treatment Market Scope
The Acute Ischemic Stroke Diagnosis and Treatment market is segmented into four notable segments based on type, surgery type, treatment, and age group.
• By Type
On the basis of type, the acute ischemic stroke diagnosis and treatment market is segmented into computed tomography (CT), magnetic resonance imaging (MRI), carotid ultrasound, cerebral angiography, electrocardiography, echocardiography, and others. The computed tomography (CT) segment dominated the largest market revenue share of 35.6% in 2024, owing to its rapid imaging capabilities critical for early stroke diagnosis.
The magnetic resonance imaging (MRI) segment is projected to register the fastest CAGR of 7.9% from 2025 to 2032, driven by its superior soft tissue contrast and non-invasive nature.
• By Surgery Type
On the basis of surgery type, the acute ischemic stroke diagnosis and treatment market is segmented into carotid endarterectomy, angioplasty, and endovascular mechanical thrombectomy. The endovascular mechanical thrombectomy segment held the highest market share of 47.2% in 2024, due to its success in large vessel occlusion treatment.
The angioplasty segment is expected to witness the fastest growth at a CAGR of 8.3% from 2025 to 2032, driven by its role in preventing recurrent ischemic strokes.
• By Treatment
On the basis of treatment, the acute ischemic stroke diagnosis and treatment market is segmented into tissue plasminogen activator, anticoagulant, antiplatelet, and antihypertensive. The tissue plasminogen activator segment accounted for the largest revenue share of 39.5% in 2024, owing to its widespread use as a first-line thrombolytic therapy.
The anticoagulant segment is anticipated to grow at the fastest CAGR of 9.1% from 2025 to 2032, driven by increasing incidence of atrial fibrillation-related strokes.
• By Age Group
On the basis of age group, the acute ischemic stroke diagnosis and treatment market is segmented into adult, geriatric, and child. The adult segment dominated with a market share of 48.3% in 2024, as lifestyle-related factors significantly contribute to stroke incidence in this group.
The geriatric segment is forecasted to grow at the highest CAGR of 8.6% from 2025 to 2032, due to the growing global elderly population and their higher vulnerability to stroke.
Acute Ischemic Stroke Diagnosis and Treatment Market Regional Analysis
- North America dominated the acute ischemic stroke diagnosis and treatment market with the largest revenue share of 38.7% in 2024, driven by increasing stroke prevalence, greater awareness of early intervention, and strong healthcare infrastructure supporting the use of advanced diagnostic and treatment technologies
- Favorable reimbursement policies, high per capita healthcare spending, and the presence of key players in the U.S. and Canada are accelerating adoption of CT, MRI, thrombectomy devices, and clot-dissolving drugs
- The expansion of telemedicine platforms and stroke care networks is also enabling faster diagnosis and treatment, contributing to reduced disability rates and boosting market growth
U.S. Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The U.S. acute ischemic stroke diagnosis and treatment market captured 71.6% of the North America market revenue in 2024, owing to a high incidence of ischemic strokes, rapid emergency response systems, and access to cutting-edge imaging and surgical procedures. Government initiatives and technological innovations in mechanical thrombectomy and neuroimaging are further fueling market expansion.
Europe Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The Europe acute ischemic stroke diagnosis and treatment market is projected to expand at a substantial CAGR throughout the forecast period, driven by aging demographics, high stroke burden, and improved stroke management pathways. Increased investments in stroke centers and emergency medical services are supporting timely imaging and treatment access across the region.
U.K. Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The U.K. acute ischemic stroke diagnosis and treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, due to national health strategies, NHS initiatives, and increasing use of digital health tools. The country's well-established infrastructure and emphasis on equitable care access support strong growth potential.
Germany Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The Germany acute ischemic stroke diagnosis and treatment market is expected to expand at a considerable CAGR during the forecast period, supported by high MRI/CT penetration, robust R&D, and hospital infrastructure. It represents 7.3% of the global market revenue in 2024 and continues to benefit from government support and innovation-focused healthcare policies.
Asia-Pacific Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The Asia-Pacific acute ischemic stroke diagnosis and treatment market is expected to grow at the fastest CAGR of 7.7% from 2025 to 2032, contributing to 18.6% of global revenue in 2024. This rapid growth is driven by rising stroke incidence, expanding healthcare access, and government investments in digital health and advanced imaging across countries such as China, Japan, and India.
Japan Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The Japan acute ischemic stroke diagnosis and treatment market accounted for 5.8% of the global market revenue in 2024 and is expected to grow at a CAGR of 7.2% from 2025 to 2032. High-tech adoption, robust insurance coverage, and a large aging population make Japan a key contributor to regional growth.
China Acute Ischemic Stroke Diagnosis and Treatment Market Insight
The China acute ischemic stroke diagnosis and treatment market held 9.3% of the global market revenue in 2024, the highest in the Asia-Pacific region, and is forecast to grow at a CAGR of 7.6% from 2025 to 2032. This growth is driven by stroke prevention programs, domestic manufacturing of cost-effective diagnostic tools, and widespread digital healthcare transformation.
Acute Ischemic Stroke Diagnosis and Treatment Market Share
The acute ischemic stroke diagnosis and treatment industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Cardinal Health (U.S.)
- Genentech, Inc. (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Medtronic (Ireland)
- Boston Scientific Corporation (U.S.)
- Bayer AG (Germany)
- GENERAL ELECTRIC (U.S.)
- Stryker (U.S.)
- Merck & Co., Inc. (U.S.)
- Hitachi High-Tech Corporation (Japan)
- Neusoft Corporation (China)
- CANON MEDICAL SYSTEMS CORPORATION (Japan)
- Teleflex Incorporated (U.S.)
- B. Braun SE (Germany)
- AliveCor India (U.S.)
- Sanofi (France)
- Johnson & Johnson Services, Inc. (U.S.)
Latest Developments in Global Acute Ischemic Stroke Diagnosis and Treatment Market
- In October 2023, Koninklijke Philips N.V., a global leader in health technology, announced a two-year partnership with the World Stroke Organization (WSO), the only global non-governmental organization dedicated to stroke. This collaboration aims to enhance access to high-quality stroke care around the world, addressing critical gaps in treatment and support. By leveraging Philips' technological expertise and WSO's advocacy, the partnership seeks to improve outcomes for stroke patients and promote better healthcare practices on a global scale
- In July 2022, Siemens Healthineers received FDA clearance for the MAGNETOM Free.Star, an affordable whole-body magnetic resonance (MR) scanner aimed at enhancing patient access to MRI technology. This innovative device is designed to provide high-quality imaging while reducing costs, making MRI more accessible to healthcare facilities and patients alike. By addressing the barriers related to MRI availability, the MAGNETOM Free.Star is poised to play a crucial role in improving diagnostic capabilities in various clinical settings
- In July 2021, Siemens Healthineers announced that the FDA granted clearance for the MAGNETOM Free.Max, an advanced high-V magnetic resonance (MR) scanner. This innovative device features a 0.55 Tesla (0.55T) field strength and integrates deep learning technologies along with sophisticated image processing capabilities. The combination of these cutting-edge features enhances imaging quality and efficiency, positioning the MAGNETOM Free.Max as a valuable tool for improving diagnostic accuracy in various medical settings
- In August 2021, Abbott announced that the U.S. Food and Drug Administration (FDA) had approved its Amplatzer Amulet Left Atrial Appendage Occluder for use in patients with atrial fibrillation (AFib) who are at risk of ischemic stroke. This innovative device is designed to effectively reduce the risk of stroke by occluding the left atrial appendage, where blood clots often form in AFib patients. With this approval, Abbott aims to enhance patient safety and outcomes for individuals vulnerable to stroke associated with AFib
- In November 2020, AstraZeneca announced that it had received approval for Brilinta (ticagrelor) in the United States to lower the risk of stroke, a major contributor to disability and mortality worldwide. This approval specifically targets patients experiencing acute ischemic stroke (with a National Institutes of Health Stroke Scale score of ≤5) or those at high risk of transient ischemic attack. By offering this innovative treatment option, AstraZeneca aims to enhance patient outcomes and address the urgent need for effective stroke prevention strategies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.