Global Activated Clotting Time Testing Market, By Product Type (Point of Care, Clinical Laboratory Analyzer), Application (Cardiovascular and Vascular Surgery, ECMO, Cardiac Catheterization Laboratories, Critical Care Units, Hemodialysis Units), Technology (Optical Detection, Laser Based Detection, Mechanical Detection, Fluorescent Based Detection), End- User (Hospitals, Clinical Laboratories, Ambulatory Surgical Centers, Academic & Research Institutes), Test (Prothrombin Time, Fibrinogen, Activated Partial Thromboplastin Time, Activated Clotting Time, D Dimer, Platelet Function, Heparin and Protamine Dose Response Test for ACT, Others) – Industry Trends and Forecast to 2030.

Activated Clotting Time Testing Market Analysis and Size
The incidence of coagulation disorders like disseminated intravascular coagulation (DIC) varies depending on the underlying condition. For example, in patients with sepsis, the incidence of DIC ranges from 20% to 50%. The annual incidence of Deep vein thrombosis (DVT) is estimated to be around 1 to 2 cases per 1,000 individuals, while the annual incidence of pulmonary embolism (PE) is approximately 0.5 to 1 case per 1,000 individuals. The incidence of haemophilia A is estimated to be around 1 in 5,000 to 10,000 male births, while the incidence of haemophilia B is lower, at approximately 1 in 30,000 male births
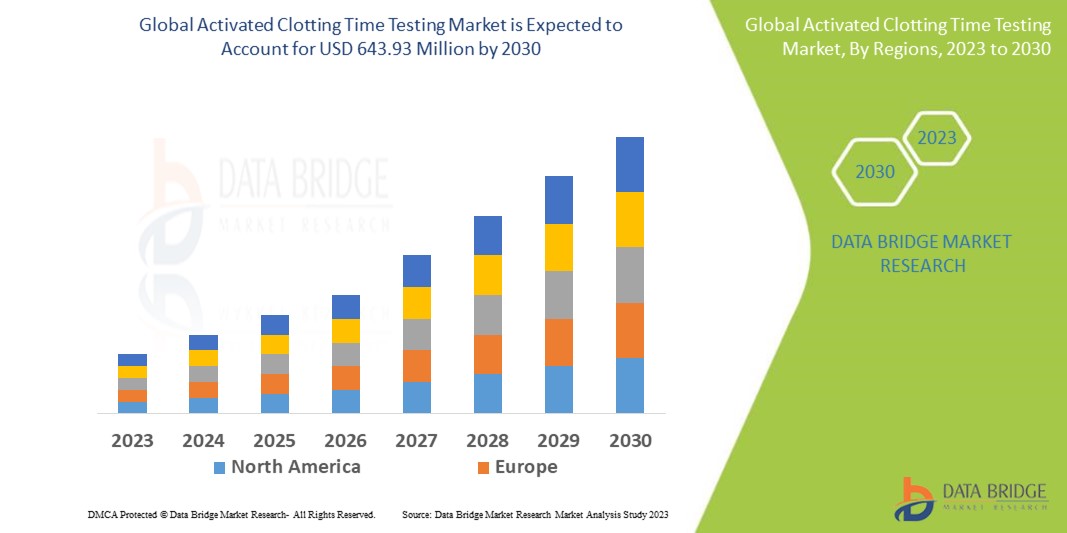
Data Bridge Market Research analyses that the global activated clotting time testing market which was USD 447.64 million in 2022, is expected to reach USD 643.93 million by 2030, and is expected to undergo a CAGR of 4.6% during the forecast period 2023-2030. This indicates the market value. “Clinical Laboratory Analyzer” dominates the product type segment of the global activated clotting time testing market owing to the growing demand for better and precise methods for diagnosis in patients. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Activated Clotting Time Testing Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, and Pricing in USD
|
|
Segments Covered
|
Product Type (Point of Care, Clinical Laboratory Analyzer), Application (Cardiovascular and Vascular Surgery, ECMO, Cardiac Catheterization Laboratories, Critical Care Units, Hemodialysis Units), Technology (Optical Detection, Laser Based Detection, Mechanical Detection, Fluorescent Based Detection), End- User (Hospitals, Clinical Laboratories, Ambulatory Surgical Centers, Academic & Research Institutes), Test (Prothrombin Time, Fibrinogen, Activated Partial Thromboplastin Time, Activated Clotting Time, D Dimer, Platelet Function, Heparin and Protamine Dose Response Test for ACT, Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East & Africa
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd (Switzerland), Siemens (Germany), Thermo Fisher Scientific (U.S.), Abbott (U.S.), Helena Laboratories Corporation (U.S.), Sysmex Corporation (Japan), NIHON KOHDEN CORPORATION (Japan), Instrumentation Laboratory India Pvt ltd (India), Medtronic (Ireland), Diagnostica Stago S.A.S (France), WerfenLife, S.A. (Spain), Laboratory Corporation of America Holdings. (U.S.), Danaher. (U.S.), Horiba Ltd. (Japan), Hycel Medical (France), Meril Life Sciences Pvt. Ltd. (India), ACON (U.S.), BIO GROUP MEDICAL SYSTEM (Canada), Beijing Succeeder Technology Inc. (China), Maccura Biotechnology Co. (China)
|
|
Market Opportunities
|
|
Market Definition
The activated clotting time (ACT) is a test that is utilized mainly to monitor high doses of unfractionated (standard) heparin therapy. Heparin is a drug that prevents the blood clotting (anticoagulant) and is generally given intravenously (IV) by injection or by continuous infusion. High doses of heparin may be given during processes that require the blood to be prevented from clotting, like the heart bypass surgery.
Global Activated Clotting Time Testing Market Dynamics
Drivers
- Increasing Prevalence of Cardiovascular Diseases
Cardiovascular diseases, such as atrial fibrillation, deep vein thrombosis, and coronary artery disease, are major contributors to morbidity and mortality worldwide. The need for accurate and timely monitoring of clotting time in patients with these conditions drives the demand for ACT testing.
- Growing Demand for Anticoagulant Therapy
Anticoagulant medications, including heparin, are commonly used in the management and prevention of blood clotting disorders. ACT testing is essential to monitor the effectiveness and safety of anticoagulant therapy, ensuring that patients are within the desired therapeutic range.
- Rising Number of Surgical Procedures
Surgical procedures, including cardiac surgeries, vascular surgeries, and orthopedic surgeries, often require anticoagulation to prevent clot formation during and after the procedure. ACT testing is crucial in guiding the administration of heparin and monitoring clotting time to prevent bleeding complications.
- Increasing Geriatric Population
The aging population is prone to various cardiovascular conditions, leading to an increased need for ACT testing. Elderly individuals often require anticoagulant therapy and close monitoring of clotting time due to their higher risk of thromboembolic events.
Opportunities
- Increasing Adoption of Point-of-Care Testing
Point-of-care testing (POCT) is gaining popularity due to its ability to provide immediate results at the patient's bedside or in outpatient settings. ACT testing at the point of care offers the opportunity for rapid and real-time monitoring of clotting time, enabling prompt adjustments in anticoagulant therapy and reducing the risk of bleeding or thrombotic events.
- Advances in Technology and Automation
Technological advancements in ACT testing devices, including the development of automated systems, provide opportunities for improved accuracy, speed, and ease of use. Automation reduces human error, streamlines workflow, and enhances efficiency in ACT testing laboratories, thereby increasing adoption and demand for ACT testing.
Restraints/Challenges
- Limited Awareness and Adoption
Despite the importance of ACT testing in monitoring clotting time, there may be limited awareness and understanding among healthcare professionals and patients. Lack of education, training, and guidelines for appropriate utilization of ACT testing can hinder its adoption, leading to underutilization or inconsistent use.
- Competition from Alternative Tests
While ACT testing is widely used in certain clinical scenarios, there are alternative tests available for assessing clotting time, such as prothrombin time (PT) testing and activated partial thromboplastin time (APTT) testing. These tests may be more commonly used or preferred in certain settings, which can limit the demand for ACT testing.
- Limitations of Current Testing Methods
Existing ACT testing methods may have limitations in terms of accuracy, precision, and reproducibility. Variations in testing protocols, inter-laboratory variability, and challenges in standardization can affect the reliability and comparability of results. Overcoming these limitations and improving the performance of ACT testing methods is a challenge for manufacturers.
- Regulatory Challenges
The global ACT testing market is subject to regulatory requirements and guidelines that vary across different regions and countries. Compliance with these regulations, obtaining necessary approvals, and adhering to quality control standards can be complex and time-consuming, posing challenges for companies operating in multiple jurisdictions.
This global activated clotting time testing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global activated clotting time testing market contact Data Bridge Market Research for an analyst brief, our team will help you make an informed market decision to achieve market growth.
Recent Developments
- In March 2021, Siemens Healthineers announced the launch of the ACTHemostasis System, a fully automated solution for ACT testing. The system offers rapid and accurate results, enhancing efficiency and workflow in coagulation testing
- In January 2021, Thermo Fisher Scientific introduced the HemosIL Liquid Heparin assay, a new reagent for ACT testing. The assay is designed to improve accuracy and precision in heparin monitoring during cardiac surgeries and other procedures
Global Activated Clotting Time Testing Market Scope
The global activated clotting time testing market is segmented on the basis of product type, application, technology, test and end users. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Point of Care
- Clinical Laboratory Analyzer
Application
- Cardiovascular and Vascular Surgery
- ECMO
- Cardiac Catheterization Laboratories
- Critical Care Units
- Hemodialysis Units
Technology
- Optical Detection
- Laser Based Detection
- Mechanical Detection
- Fluorescent Based Detection
Test
- Prothrombin Time
- Fibrinogen
- Activated Partial Thromboplastin Time
- Activated Clotting Time
- D Dimer
- Platelet Function
- Heparin and Protamine Dose Response Test for ACT
- Others
End Users
- Hospitals
- Clinical Laboratories
- Ambulatory Surgical Centres
- Academic & Research Institutes
Global Activated Clotting Time Testing Market Regional Analysis/Insights
The global activated clotting time testing market is analyzed and market size insights and trends are provided by country, product type, application, technology, test and end users as referenced above.
The countries covered in the global activated clotting time testing market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East & Africa.
North America dominates the global activated clotting time testing market because of the strong base of healthcare facilities, the strong presence of major players in the market, the extraordinary healthcare infrastructure, and the large pool of people having risk of bleeding or thrombotic complications.
Asia-Pacific is expected to witness significant growth during the forecast period of 2023 to 2030 due to the increase in government initiatives to promote healthcare, the rising health awareness among the people and growing demand for advanced medical technology for diagnosis and treatment procedures, the large population pool, and the growing demand for quality healthcare in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The global activated clotting time testing market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the global activated clotting time testing market, the impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the global activated clotting time testing market. The data is available for historic period 2015-2020.
Competitive Landscape and Global Activated Clotting Time Testing Market Share Analysis
The global activated clotting time testing market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the companies' focus related to the global activated clotting time testing market.
Some of the major players operating in the global activated clotting time testing market are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Siemens (Germany)
- Thermo Fisher Scientific (U.S.)
- Abbott (U.S.)
- Helena Laboratories Corporation (U.S.)
- Sysmex Corporation (Japan)
- NIHON KOHDEN CORPORATION (Japan)
- Instrumentation Laboratory India Pvt ltd (India)
- Medtronic (Ireland)
- Diagnostica Stago S.A.S (France)
- WerfenLife, S.A. (Spain)
- Laboratory Corporation of America Holdings. (U.S.)
- Danaher. (U.S.)
- Horiba Ltd. (Japan)
- Hycel Medical (France)
- Meril Life Sciences Pvt. Ltd. (India)
- ACON (U.S.)
- BIO GROUP MEDICAL SYSTEM (Canada)
- Beijing Succeeder Technology Inc. (China)
- Maccura Biotechnology Co. (China)
SKU-

