Global Acetaminophen Paracetamol Market
Market Size in USD Million
CAGR :
% 
 USD
796.79 Million
USD
1,124.48 Million
2024
2032
USD
796.79 Million
USD
1,124.48 Million
2024
2032
| 2025 –2032 | |
| USD 796.79 Million | |
| USD 1,124.48 Million | |
|
|
|
|
Acetaminophen (Paracetamol) Market Analysis
The Acetaminophen (Paracetamol) market is experiencing steady growth, driven by its widespread use as an analgesic and antipyretic for treating pain and fever. The rising prevalence of chronic diseases, flu, and viral infections has increased demand for paracetamol-based medications, both in prescription and over-the-counter (OTC) forms. In addition, advancements in drug formulations, combination therapies, and extended-release variants have enhanced the effectiveness and market reach of acetaminophen. The integration of paracetamol with ibuprofen in pain management solutions, such as Maxigesic IV, highlights a shift towards non-opioid alternatives to address the growing opioid crisis. The market is further fueled by regulatory approvals, increasing healthcare spending, and technological advancements in pharmaceutical manufacturing. Companies are focusing on sustainable production processes, including vegan-certified formulations such as Paraveganio. Moreover, expanding distribution networks and licensing agreements in regions such as Europe, South America, and Asia-Pacific are enhancing global accessibility. With ongoing innovations in drug delivery systems, compliance with safety regulations, and increased consumer awareness, the acetaminophen market is poised for substantial growth in the coming years.
Acetaminophen (Paracetamol) Market Size
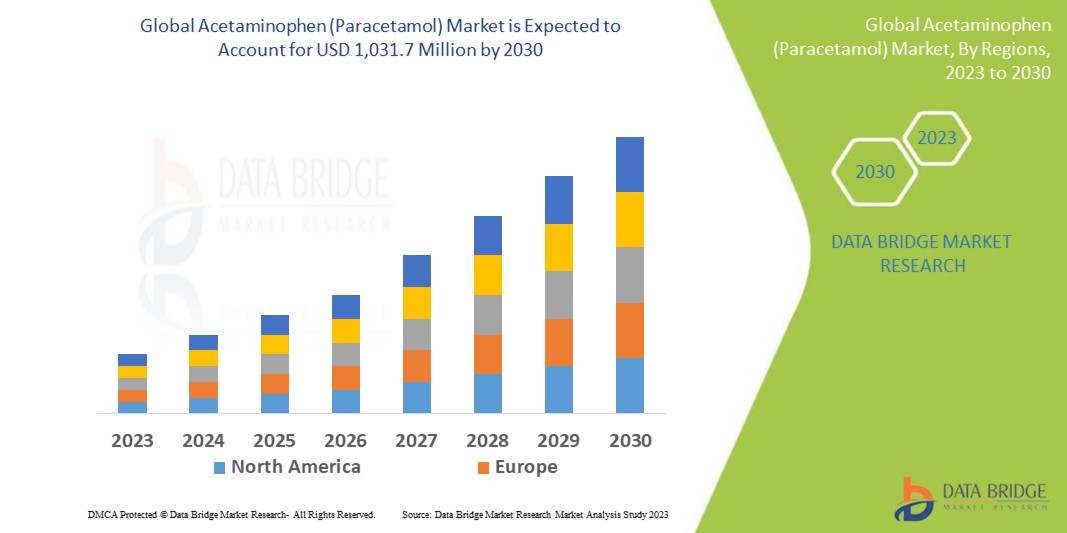
The global acetaminophen (paracetamol) market size was valued at USD 796.79 million in 2024 and is projected to reach USD 1124.48 million by 2032, with a CAGR of 4.40 % during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Acetaminophen (Paracetamol) Market Trends
“Growing Adoption of Combination Drug Formulations”
One key trend shaping the Acetaminophen (Paracetamol) market is the growing adoption of combination drug formulations to enhance pain management efficacy and reduce dependency on opioids. Pharmaceutical companies are increasingly developing dual-action therapies, such as Maxigesic IV, which combines paracetamol and ibuprofen to provide superior postoperative pain relief. This trend aligns with the global push for non-opioid alternatives, particularly in regions such as North America and Europe, where stringent regulations are in place to curb opioid overuse. In addition, advancements in intravenous (IV) formulations are expanding the use of acetaminophen in hospital settings, offering faster and more effective pain relief. For instance, the FDA-approved Maxigesic IV is gaining traction in the U.S. and European markets, contributing to the market’s growth. As demand for safer, non-addictive pain relievers rises, the integration of acetaminophen with complementary analgesics is expected to drive further expansion in the industry.
Report Scope and Acetaminophen (Paracetamol) Market Segmentation
|
Attributes |
Acetaminophen (Paracetamol) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Farmson Basic Drugs Private Limited (India), Granules India Limited (India), Hebei Jiheng Pharmaceutical Co., Ltd (China)., Mallinckrodt (U.K.), Zhengzhou Sino Chemical Products Co., Ltd (China), Haihang Industry (China), and BOC Sciences (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Acetaminophen (Paracetamol) Market Definition
Acetaminophen, also known as paracetamol, is a widely used analgesic (pain reliever) and antipyretic (fever reducer). It works by inhibiting prostaglandin synthesis in the brain, which helps reduce pain and regulate body temperature. Unlike nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen does not have significant anti-inflammatory properties and is considered gentler on the stomach, making it suitable for individuals with gastric sensitivity or ulcers.
Acetaminophen (Paracetamol) Market Dynamics
Drivers
- Rising Prevalence of Chronic Diseases and Pain Management Needs
The increasing burden of chronic diseases, including arthritis, migraines, and musculoskeletal disorders, has significantly driven the demand for acetaminophen-based medications. With millions of people suffering from long-term pain conditions, the need for safe, effective, and well-tolerated analgesics has surged. Acetaminophen (paracetamol) is preferred over NSAIDs for patients with gastric sensitivity or those at risk of gastrointestinal ulcers. For instance, in the U.S., where approximately 54 million adults suffer from arthritis, acetaminophen is widely recommended as a first-line pain relief treatment. Furthermore, the aging population contributes to increased consumption, as older adults are more susceptible to joint pain and inflammation-related conditions. This growing demand solidifies acetaminophen’s role in the global pain management market, positioning it as a key driver of industry growth.
- Expanding Healthcare Access and Over-the-Counter (OTC) Availability
The easy accessibility of acetaminophen across pharmacies, supermarkets, and online platforms has significantly propelled its market growth. As an OTC medication, paracetamol does not require a prescription in most countries, making it a go-to option for consumers seeking quick pain relief. Many governments and healthcare organizations are promoting affordable pain management solutions, increasing the global demand for cost-effective drugs such as acetaminophen. For instance, in India, the government’s Jan Aushadhi Scheme promotes the availability of generic paracetamol at subsidized rates, ensuring wider accessibility to lower-income groups. Moreover, the rise of e-commerce platforms, such as Amazon Pharmacy and online drugstores, has further simplified consumer access, allowing patients to purchase medications conveniently from home. This shift towards digital healthcare solutions and online pharmaceutical sales has strengthened the market penetration of acetaminophen, making OTC availability a major driver in the industry’s expansion.
Opportunities
- Growth in Combination Drug Formulations
The rising demand for effective and opioid-free pain management solutions has led pharmaceutical companies to develop combination drug formulations featuring acetaminophen (paracetamol) alongside complementary analgesics. These dual-action therapies enhance pain relief efficacy, prolong therapeutic effects, and reduce opioid dependency, which is a growing concern worldwide. For instance, Maxigesic IV, a combination of paracetamol and ibuprofen, has gained popularity for postoperative pain management due to its superior pain-relieving properties. The increasing adoption of such combination formulations is driving regulatory approvals and expanding the market reach of acetaminophen-based medications. In addition, with the FDA and European Medicines Agency (EMA) supporting non-opioid alternatives, pharmaceutical companies are leveraging this opportunity to introduce innovative pain relief solutions, further strengthening the global acetaminophen market.
- Increasing Awareness and Government Initiatives
Growing public awareness regarding the safe use of painkillers and the dangers of opioid addiction has significantly boosted the demand for safer alternatives such as acetaminophen. Governments and regulatory bodies worldwide are implementing strict opioid regulations and promoting responsible analgesic use, creating a favorable market environment for non-opioid pain relievers. For instance, the U.S. Centers for Disease Control and Prevention (CDC) has issued opioid prescribing guidelines, encouraging healthcare providers to recommend acetaminophen as a first-line treatment for mild to moderate pain. Similarly, the World Health Organization (WHO) recognizes paracetamol as an essential medicine, reinforcing its global demand and accessibility. As governments continue to introduce public health campaigns, stricter opioid regulations, and prescription monitoring programs, the acetaminophen market stands to benefit from increased consumer preference for safer, widely available, and cost-effective pain management solutions.
Restraints/Challenges
- Regulatory and Compliance Challenge
The Acetaminophen (Paracetamol) market is heavily regulated due to concerns over potential misuse and health risks associated with overdose. Regulatory bodies such as the U.S. FDA, European Medicines Agency (EMA), and the World Health Organization (WHO) have implemented strict guidelines regarding dosage limits, labeling requirements, and manufacturing standards. For instance, the FDA has limited the maximum dosage of acetaminophen in prescription combination drugs to 325 mg per tablet to reduce the risk of liver toxicity. In addition, some countries, including Australia and the U.K., have imposed restrictions on over-the-counter (OTC) sales, requiring consumers to purchase acetaminophen in limited quantities to prevent accidental overdoses. While these regulations help protect public health, they increase compliance costs for pharmaceutical manufacturers, requiring them to conduct rigorous safety studies, update product labels, and navigate complex approval processes. This presents a major market challenge, as companies must allocate significant resources to meet regulatory demands, delaying new product launches and increasing production costs.
- Safety and Health Risks
One of the biggest challenges in the acetaminophen market is its potential for liver toxicity and overdose, which can lead to severe health complications. Acetaminophen overdose is a leading cause of acute liver failure in the U.S., accounting for nearly 50% of all cases, according to data from the American Association for the Study of Liver Diseases (AASLD). Even small overdoses over time can lead to chronic liver damage, making dosage monitoring critical. In addition, combining acetaminophen with alcohol or certain prescription drugs can increase toxicity risks, further complicating its safe use. A notable case occurred in 2011 when the FDA issued a warning about the risk of severe liver injury associated with high doses of acetaminophen, leading to a push for reduced dosages in combination medications. These health risks create a significant market challenge, as pharmaceutical companies must invest in public awareness campaigns, improved packaging with overdose warnings, and the development of safer formulations, all of which add to operational costs.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Acetaminophen (Paracetamol) Market Scope
The market is segmented on the basis of type, product type, application, and route of administration. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Veterinary Drug Grade
- Acetaminophen Grade
Product Type
- Tablet
- Capsule
- Liquid Suspension
- Powder
Application
- Chemical Industry
- Pharma Industry
- Others
Route of Administration
- Oral
- Rectal
- Intravenous
Acetaminophen (Paracetamol) Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, product type, application, and route of administration as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to dominate the market during the forecast period, driven by rising healthcare expenditure and the widespread availability of cost-effective medications. The region's strong healthcare infrastructure and favorable reimbursement policies further support market expansion. In addition, the increasing prevalence of chronic pain conditions and the growing demand for effective pain management solutions are fueling the adoption of pharmaceutical products. Heightened awareness among patients and healthcare providers about advanced treatment options is also contributing to market growth.
Asia-Pacific is emerging as the fastest growing market due to the rising burden of chronic diseases and the growing demand for effective pain management solutions. The increasing prevalence of fever, headaches, and migraines, along with a surge in healthcare awareness, is driving market expansion. In addition, the presence of major pharmaceutical companies and their efforts to introduce innovative and affordable medications are accelerating growth in the region. The expanding healthcare infrastructure and higher accessibility to pain relief treatments further contribute to the market's strong potential.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Acetaminophen (Paracetamol) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Acetaminophen (Paracetamol) Market Leaders Operating in the Market Are:
- Farmson Basic Drugs Private Limited (India)
- Granules India Limited (India)
- Hebei Jiheng Pharmaceutical Co., Ltd (China)
- Mallinckrodt (U.K.)
- Zhengzhou Sino Chemical Products Co., Ltd (China)
- Haihang Industry (China)
- BOC Sciences (U.S.)
Latest Developments in Acetaminophen (Paracetamol) Market
- In July 2024, Hyloris Pharmaceuticals entered into an agreement with Halex Istar for the licensing and distribution of Maxigesic IV in Brazil, expanding access to this non-opioid pain management solution in one of South America’s largest markets. Maxigesic IV, a combination of paracetamol and ibuprofen, aims to provide enhanced post-operative pain relief while reducing opioid dependency. This collaboration aligns with Hyloris’s strategy to optimize existing drugs for unmet medical needs, with the company expecting revenue growth from royalties and milestone payments
- In January 2023, Hyloris Pharmaceuticals SA partnered with Salus Pharmaceuticals for the exclusive licensing and distribution of Maxigesic IV across nine European countries. This dual-action, non-opioid IV pain treatment, combining paracetamol and ibuprofen, has already been registered in five of these markets, with product launches planned later in 2023. This move supports Hyloris’s vision of repurposing existing drugs and expanding access to innovative, non-opioid pain management solutions
- In April 2022, IOL Chemicals and Pharmaceuticals began commercial production of paracetamol with an installed capacity of 1,800 MTPA, incorporating backward integration of Para Amino Phenol (PAP). This initiative strengthened the company’s production capabilities. In addition, in response to the current market demand, the company is expanding its paracetamol production capacity from 1,800 MTPA to 3,600 MTPA
- In March 2022, Paraveganio became one of the first medicinal products globally to be registered with The Vegan Society's Vegan Trademark. This certified vegan drug contains 500mg of paracetamol, offering a plant-based alternative for pain relief
- In November 2021, Hyloris Pharmaceuticals SA announced that the U.S. Food and Drug Administration (FDA) accepted the New Drug Application (NDA) for Maxigesic IV, a unique combination of 1000 mg paracetamol and 300 mg ibuprofen for postoperative pain relief. This regulatory milestone allowed the company to broaden the applications of its product and expand its customer base
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.