Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market Analysis and Insights
The growing prevalence of infectious and respiratory diseases like COVID-19 and acute respiratory distress syndrome and the wide focus on vaccine developments and therapeutic and diagnostic products for these conditions have enhanced the market demand. The advancement in technology for easy supply of products and fast manufacturing facilities are also attributing to the growth of the market. The major market players are highly focused on product launches and approvals during this crucial period. In addition, the government and regulatory bodies are supporting market players with product approval due to surging emergence.
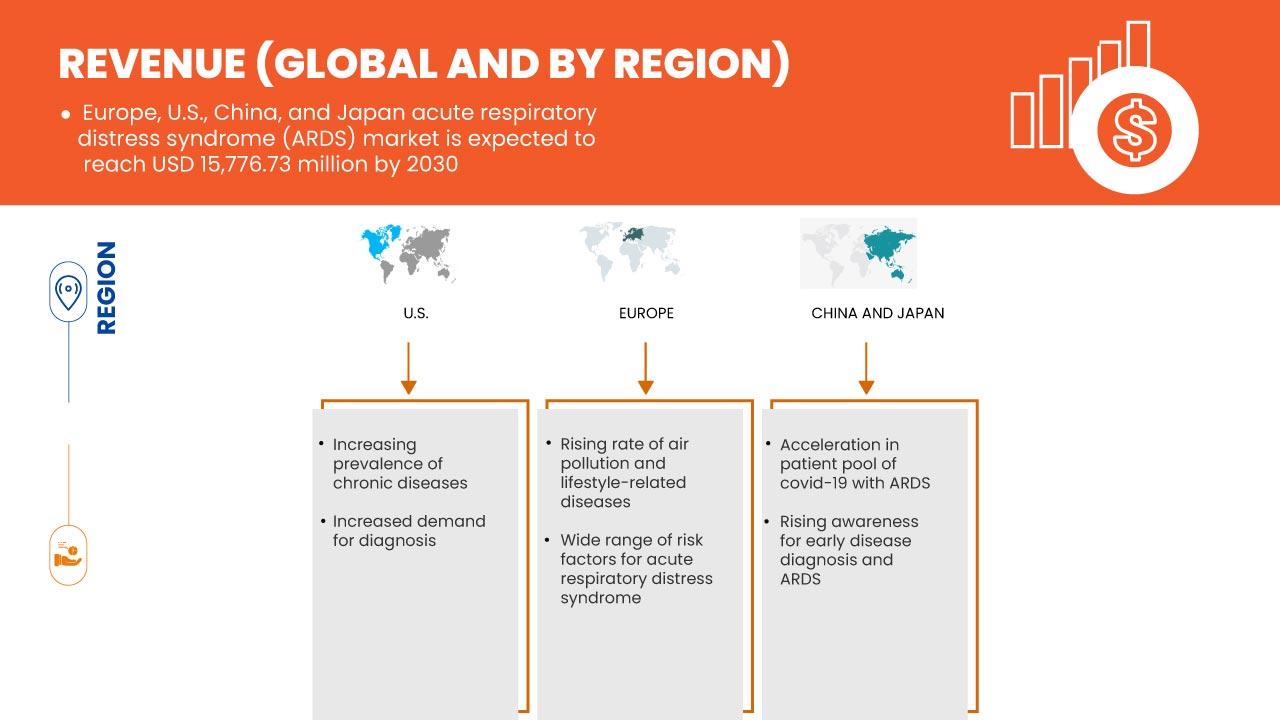
The Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market is supportive and aims to reduce the disease, thereby improving the recovery and performance of individuals. Data Bridge Market Research analyzes that Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market will grow at a CAGR of 9.9% during the forecast period of 2023 to 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2020-2015) |
|
Quantitative Units |
Revenue in Million, Pricing in USD |
|
Segments Covered |
By Cause (Coronavirus Disease 2019 (COVID-19), Sepsis, Inhalation of Harmful Substances, Severe Pneumonia and Others), Type (Diagnosis and Treatment), Route of Administration (Oral, Parenteral and Others), End User (Hospitals, Specialty Clinics, Home Healthcare and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy and Online Pharmacy) |
|
Country Covered |
U.S., Japan, China, Germany, U.K., Italy, France, Spain, Switzerland, Russia, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Netherlands and the Rest of Europe |
|
Market Players Covered |
Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB., and EUROSETS, among others |
Market Definition
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury that allows fluid to leak into the lungs. Most people who get ARDS are hospitalized for trauma or illness like COVID-19. The syndrome usually occurs when fluids build up in the lungs' tiny, elastic air sacs called alveoli. This fluid build-up causes less oxygen to reach the bloodstream. This deprives the organs of getting enough oxygen for their normal function. People with other illness develops ARDS within a few hours to days after the precipitating injury or infection. The risk of death increases with age, and depending on the severity of the illness, patients surviving the syndrome becomes hard. Severe illness or injury causing damage to the membrane sacs of the lungs leads to ARDS. The most common underlying causes for the said diseases include sepsis, inhalation of harmful substances, severe pneumonia, head, chest or another major injury, coronavirus disease 2019 (COVID-19) and others.
Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market Dynamics
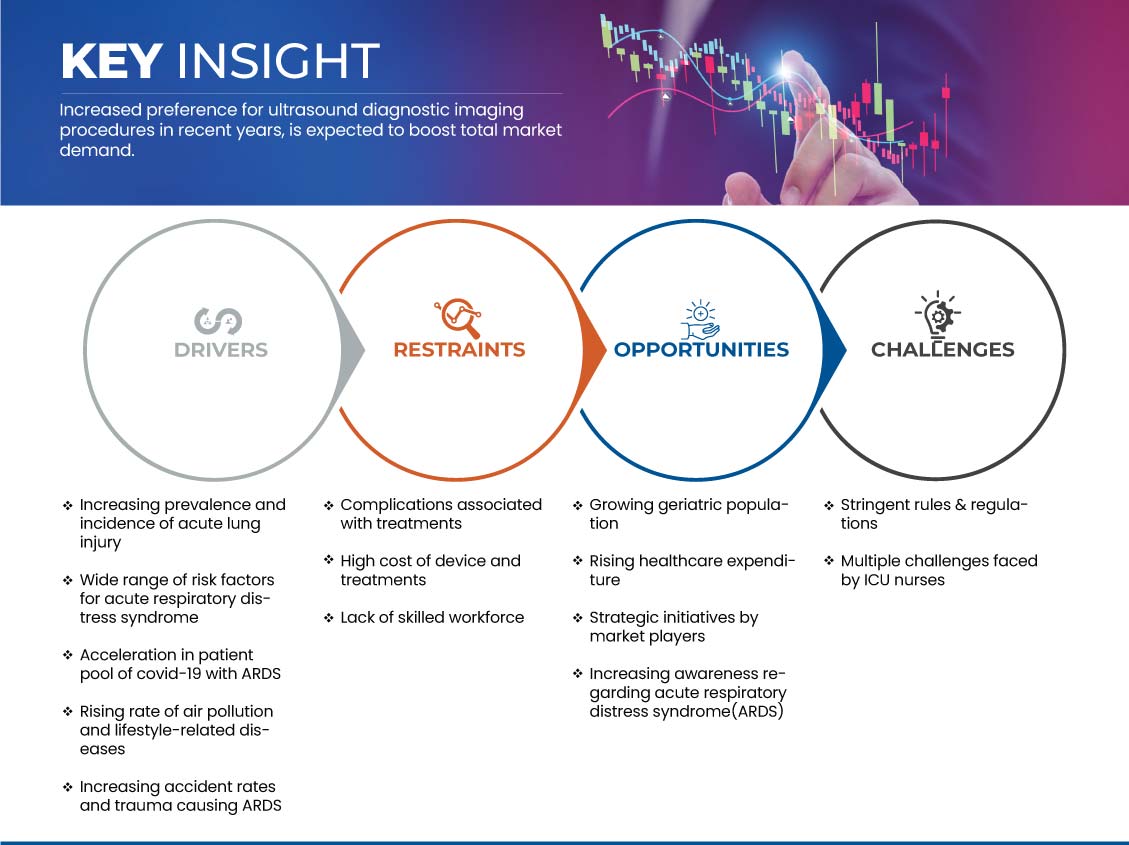
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of these are discussed in detail below:
Drivers
- Increasing prevalence and incidence of acute lung injury
Patients with acute lung injuries are being widely reported due to numerous factors like the increasing aging population and the rising number of patients with sepsis and pneumonia. However, most people are diagnosed with lung injuries and acute respiratory distress syndrome only in the late stages. The said disease is a rapidly progressive condition occurring in patients with damaged lungs, causing bodily fluids to leak. The number of acute respiratory distress syndrome cases and lung injuries is increasing due to the emergence of various respiratory illness-causing viruses in recent years, like COVID-19.
Thereby, the incidence and prevalence of acute respiratory distress syndrome keep increasing. The disease has been widely recognized as a major clinical problem worldwide, carrying high morbidity and mortality burden. Hence, increasing prevalence and incident rates of acute lung injuries and accompanied acute respiratory distress syndrome is expected to drive the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market.
- Wide range of risk factors for Acute Respiratory Distress Syndrome (ARDS)
There is an enormous range of risk factors being reported for acute respiratory distress syndrome. There are environmental and individual risk factors involved with the syndrome. Patients with ARDS suffer from varying degrees of pulmonary artery vasoconstriction that cause the problem of getting enough oxygen into the blood. Thus, they usually need a ventilator to breathe. ARDS causes high mortality and ameliorates this deadly condition. Sepsis syndrome with multiple organ failure is the most common cause of death, while respiratory failure is second. Moreover, the severity of ARDS is associated significantly with the mortality rate among critically ill COVID-19 patients.
ARDS can be induced by multiple causes, including trauma. Risk factors for ARDS after multiple trauma include traumatic brain and chest injury, severity and duration of shock, number of transfused blood products, and infused crystalloids.
Opportunity
- Increasing awareness regarding acute respiratory distress syndrome (ARDS)
Since acute respiratory distress syndrome has multiple different causes, it is usually ignored under common causes of death. The requirement of advanced technical treatment and proper awareness of the condition can substantially decline acute respiratory distress syndrome incidence. As timely diagnosis and prevention are crucial to preventing or recovering faster, the public's attention is most important. Current governments and organizations have broadened the scope of lung injury research to include primary prevention of acute respiratory distress syndrome and reduce the morbidity or mortality rate for the syndrome.
The initiatives that started back are still aiding in the prevention of severe lung infections like ARDS and support biotechnology and pharmaceutical companies to innovate their research for new advancements in treatments. Though there is no proper or specific cure for ARDS, few associations are trying to increase awareness of the syndrome and aid patients in receiving timely healing for their lungs.
Such novel initiative programs and supportive care units started by various healthcare and lung care associations are increasing awareness among people regarding the cause and proper disease management on time. Thereby, increasing awareness regarding ARDS through diverse associations enhances the opportunity for future Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market growth.
Restraint/Challenge
- High cost of devices and treatments
Though acute respiratory distress syndrome is getting a wide range of advanced treatment options, the longer treatment cost is quite difficult for average-income people to afford. The utilization of critical care and intensive care unit services is increasing worldwide, and its expensive cost is a major concern in the current healthcare system. Patients with acute respiratory distress syndrome are commonly required to have long hospitalizations with frequent monetarization and ventilation usage, consuming a significant amount of healthcare resources. Due to this, most patients who cannot afford a long-term stay get discharged in the initial stages of treatment. However, this increases the possibilities and susceptibilities for new complications in infections, which demands additional healthcare resources and treatment.
Post-COVID-19 Impact on Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market
COVID-19 has positively affected the market's growth as there is a rise in the demand for acute respiratory distress syndrome in the region. During the phase of COVID-19, it was indicated that several cases are asymptomatic, while 20% of COVID-19 cases follow a severe course, necessitating hospitalization. Severe cases of COVID-19 disease will ultimately lead to ARDS and pneumonia. This has been proven to be fatal for infected individuals. As ARDS shows the lung defect by damaging the alveoli, which are tiny air sacs in the lungs, the same level of defect has been observed in COVID-19 patients. This leads to a sudden influx of liquid, causing pneumonia. Hence COVID-19 has positively impacted this market.
Recent Developments
- In May 2021, Medtronic launched the SonarMed airway monitoring system. The system utilizes acoustic technology to check for endotracheal tube obstruction. This has helped the company to increase its product portfolio
- In July 2020, F. Hoffman-La Roche Ltd launched SARS-CoV-2 rapid antibody test. The test was launched in partnership with SD Biosenseor, Inc. This has helped the company to increase its product portfolio
Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market Scope
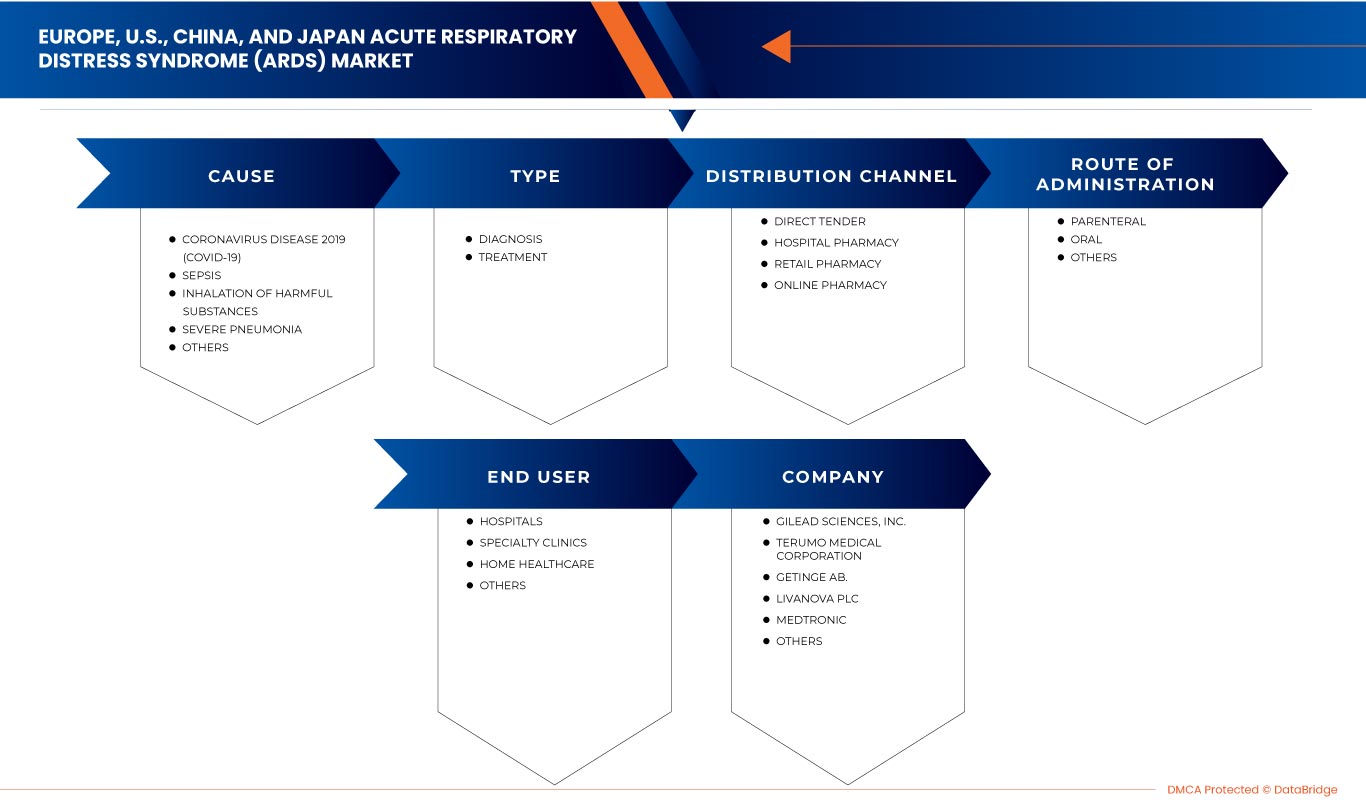
Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is categorized into five segments based on cause, type, route of administration, end-user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Cause
- Coronavirus Disease 2019 (COVID-19)
- Sepsis
- Inhalation Of Harmful Substances
- Severe Pneumonia
- Others
Based on the cause, the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is segmented into coronavirus disease 2019 (COVID-19), sepsis, inhalation of harmful substances, severe pneumonia, and others.
Type
- Diagnosis
- Treatment
Based on type, the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is segmented into diagnosis and treatment.
Route of Administration
- Oral
- Parenteral
- Others
Based on route of administration, the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is segmented into oral, parenteral, and others.
End User
- Hospitals
- Specialty Clinics
- Home Healthcare
- Others
Based on end user, the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is segmented into hospitals, specialty clinics, home healthcare and others.
Distribution Channel
- Direct Tender
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Based on distribution channel, the Europe, U.S., China and Japan acute respiratory distress syndrome (ARDS) market is segmented into direct tender, hospital pharmacy, retail pharmacy and online pharmacy.
Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market Country Analysis/Insights
Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market is analyzed, and market size insights and trends are provided by the cause, type, route of administration, end user, and distribution channel as referenced above.
The country covered in the Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market are U.S., Japan, China, Germany, U.K., Italy, France, Spain, Switzerland, Russia, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Netherlands and the Rest of Europe.
U.S. acute respiratory distress syndrome (ARDS) market is expected to grow due to an increase in the prevalence of acute lung injuries as well as a rise in the number of the patient pool of COVID-19 with ARDS. These are the key contributing factors which is expected to boost the growth of the market in the country.
The country section of the report also provides individual market-impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as downstream and upstream value chain analysis, technical trends, porter's five forces analysis, and case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of brands and their challenges faced due to large or scarce competition from local and domestic brands and the impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe, U.S., China, and Japan Acute Respiratory Distress Syndrome (ARDS) Market Share Analysis
Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market competitive landscape provides details of the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the company's focus on the Europe, U.S., China, and Japan acute respiratory distress syndrome (ARDS) market.
Some of the major players operating in the market are Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB., and EUROSETS, among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 ETIOLOGY BY GEOGRAPHY
4.3.1 ETIOLOGY IN U.S.
4.3.2 ETIOLOGY IN EUROPE
4.3.3 ETIOLOGY IN CHINA
4.3.4 ETIOLOGY IN JAPAN
4.4 ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) HEALTHCARE COST PER PATIENT BY GEOGRAPHY
4.5 INSURANCE REIMBURSEMENT
4.5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
4.5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
4.5.3 ABBOTT CODING GUIDE FOR ECMO
4.5.4 CENTRAL GOVERNMENT HEALTH SCHEME (CGHS)
4.5.5 CERN HEALTH INSURANCE SCHEME
4.5.6 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
4.5.7 AMERICAN HOSPITAL ASSOCIATION
4.5.8 CONCLUSION
4.6 PIPELINE ANALYSIS
5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
5.1 REGULATION IN U.S.:
5.2 LABELING OF MODIFIED DEVICES
5.3 REGULATION IN EUROPE:
5.4 REGULATION IN CHINA:
5.5 REGULATION IN JAPAN:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
6.1.2 WIDE RANGE OF RISK FACTORS FOR ACUTE RESPIRATORY DISTRESS SYNDROME
6.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
6.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
6.1.5 INCREASING ACCIDENT RATES AND TRAUMA-CAUSING ARDS
6.2 RESTRAINTS
6.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
6.2.2 HIGH COST OF DEVICE AND TREATMENTS
6.2.3 LACK OF SKILLED WORKFORCE
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISING HEALTHCARE EXPENDITURE
6.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.4 INCREASING AWARENESS REGARDING ACUTE RESPIRATORY DISTRESS SYNDROME(ARDS)
6.4 CHALLENGES
6.4.1 STRINGENT RULES & REGULATIONS
6.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
7.1 OVERVIEW
7.2 CORONAVIRUS DISEASE 2019 (COVID-19)
7.3 SEPSIS
7.4 INHALATION OF HARMFUL SUBSTANCES
7.5 SEVERE PNEUMONIA
7.6 OTHERS
8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
8.1 OVERVIEW
8.2 DIAGNOSIS
8.2.1 IMAGING TESTS
8.2.1.1 CHEST X-RAY
8.2.1.2 CT SCAN
8.2.1.3 ULTRASOUND
8.2.1.4 OTHERS
8.2.2 BLOOD TEST
8.2.3 RESPIRATORY RATE
8.2.4 SPO2 TEST
8.2.5 OTHERS
8.3 TREATMENT
8.3.1 MECHANICAL VENTILATION
8.3.1.1 HIGH-FLOW NASAL O2
8.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
8.3.1.3 CONTINUOUS POSITIVE AIRWAY PRESSURE
8.3.1.4 PRONE POSITIVE VENTILATION
8.3.1.5 OTHERS
8.3.2 CORTICOSTEROIDS
8.3.2.1 METHYLPREDNISOLONE
8.3.2.2 DEXAMETHASONE
8.3.2.3 OTHERS
8.3.3 ANTIVIRAL MEDICATION
8.3.3.1 REMDESIVIR
8.3.3.2 COMBINATION DRUGS
8.3.3.3 OTHERS
8.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
8.3.5 TOCILIZUMAB
8.3.6 OTHERS
9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.2.1 INTRAVENOUS
9.2.2 INTRAMUSCULAR
9.3 ORAL
9.4 OTHERS
10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOME HEALTHCARE
10.5 OTHERS
11 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 HOSPITAL PHARMACY
11.4 RETAIL PHARMACY
11.5 ONLINE PHARMACY
12 EUROPE
12.1 GERMANY
12.2 FRANCE
12.3 U.K.
12.4 ITALY
12.5 SPAIN
12.6 TURKEY
12.7 HUNGARY
12.8 NETHERLANDS
12.9 SWITZERLAND
12.1 AUSTRIA
12.11 LITHUANIA
12.12 POLAND
12.13 RUSSIA
12.14 IRELAND
12.15 NORWAY
12.16 REST OF EUROPE
13 EUROPE, US, CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: U.S.
13.2 COMPANY SHARE ANALYSIS: EUROPE
13.3 COMPANY SHARE ANALYSIS: JAPAN
13.4 COMPANY SHARE ANALYSIS: CHINA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 GILEAD SCIENCES INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUS ANALYSIS
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENT
15.2 TERUMO CORPORATION
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUS ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 GETINGE AB
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUS ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 LIVANOVA PLC
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENTS
15.5 MEDTRONIC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENTS
15.6 ALUNG TECHNOLOGIES, INC
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 ARMSTRONG MEDICAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 BESMED HEALTH BUSINESS CORP.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 DRÄGERWERK AG & CO. KGAA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 EUROSETS
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 F. HOFFMANN-LA ROCHE LTD
15.11.1 COMPANY SNAPSHOT
15.11.2 RECENT ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.12 FISHER & PAYKEL HEALTHCARE LIMITED
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 FRESENIUS SE & CO. KGAA
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUS ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HAMILTON MEDICAL
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 NICE NEOTECH MEDICAL SYSTEMS PVT.LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NIPRO
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUS ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 PFIZER INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUS ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 RESMED
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 SMITHS MEDICAL
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUS ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 HEALTHCARE COST FOR ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) ON THE BASIS OF SEVERITY BY COUNTRY IS GIVEN BELOW IN USD:
TABLE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 3 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
TABLE 4 REGULATION FOR THE USE OF VENTILATOR AND ANESTHESIA GAS MACHINE BREATHING CIRCUIT DEVICES
TABLE 5 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 7 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 8 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 14 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 CHINA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 16 JAPAN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 17 EUROPE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 18 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 CHINA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 20 JAPAN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030(USD MILLION)
TABLE 22 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 23 CHINA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 24 JAPAN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 27 CHINA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 EUROPE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 CHINA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 33 EUROPE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 34 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 CHINA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 36 JAPAN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 40 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 EUROPE PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 U.S. PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 43 CHINA PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 44 JAPAN PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 45 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 49 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 50 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 54 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 55 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 56 GERMANY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 57 GERMANY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 58 GERMANY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 59 GERMANY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 60 GERMANY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 61 GERMANY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 62 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 63 GERMANY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 64 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 66 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 67 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 FRANCE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 69 FRANCE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 FRANCE PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 76 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 79 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.K. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.K. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.K. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.K. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.K. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.K. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 87 U.K. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 88 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 89 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 90 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 91 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 92 ITALY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 93 ITALY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 ITALY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 95 ITALY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 96 ITALY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 97 ITALY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 98 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 99 ITALY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 100 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 103 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 104 SPAIN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 105 SPAIN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 106 SPAIN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 107 SPAIN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 108 SPAIN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 109 SPAIN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SPAIN PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 115 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 TURKEY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 TURKEY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 TURKEY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 TURKEY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 120 TURKEY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 121 TURKEY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 122 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 123 TURKEY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION
TABLE 124 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 125 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 126 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 127 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 128 HUNGARY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 129 HUNGARY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 HUNGARY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 HUNGARY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 132 HUNGARY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 HUNGARY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 135 HUNGARY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 136 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 137 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 138 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 139 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 140 NETHERLANDS DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 141 NETHERLANDS IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 142 NETHERLANDS TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 143 NETHERLANDS ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 NETHERLANDS CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 145 NETHERLANDS MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 146 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 NETHERLANDS PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 148 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 149 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 150 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 151 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 SWITZERLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 SWITZERLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 SWITZERLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 SWITZERLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 156 SWITZERLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 157 SWITZERLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 158 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 SWITZERLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 163 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 164 AUSTRIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 165 AUSTRIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 AUSTRIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 167 AUSTRIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 AUSTRIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 AUSTRIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 171 AUSTRIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 172 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 173 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 174 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 175 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 LITHUANIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 177 LITHUANIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 178 LITHUANIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 LITHUANIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 180 LITHUANIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 181 LITHUANIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 183 LITHUANIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 184 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 185 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 186 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 187 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 POLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 POLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 POLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 191 POLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 192 POLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 193 POLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 194 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 POLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 197 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 198 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 199 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 200 RUSSIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 201 RUSSIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 202 RUSSIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 203 RUSSIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 RUSSIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 RUSSIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 207 RUSSIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 208 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 209 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 210 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 211 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 212 IRELAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 213 IRELAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 214 IRELAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 215 IRELAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 216 IRELAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 217 IRELAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 218 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 219 IRELAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 220 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 221 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 222 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 223 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 224 NORWAY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 225 NORWAY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 226 NORWAY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 227 NORWAY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 228 NORWAY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 229 NORWAY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 231 NORWAY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 232 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 233 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 234 REST OF EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN THE PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 13 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S.ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 14 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 15 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 16 MOST COMMON PRIMARY CAUSES OF DEATH IN ARDS PATIENTS IN U.S. COUNTRY
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 18 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 19 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 21 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 26 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 27 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 28 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 29 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 30 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 31 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 32 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 33 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 34 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 35 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 36 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 39 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 40 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 41 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 42 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 43 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 44 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 45 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 46 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 47 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 49 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 50 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 51 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 52 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 54 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 55 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 56 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 57 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 58 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 59 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 60 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 61 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 62 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 63 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 64 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 65 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 66 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 67 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 68 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 69 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 70 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 71 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 72 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 73 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 74 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 75 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 76 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 77 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 78 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 79 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 80 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 81 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 82 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 83 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 84 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 85 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 86 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 87 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 88 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 89 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 90 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 91 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 92 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 93 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 94 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 95 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 96 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 97 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 98 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SNAPSHOT (2022)
FIGURE 99 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022)
FIGURE 100 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2023 & 2030)
FIGURE 101 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022 & 2030)
FIGURE 102 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: CAUSE (2023-2030)
FIGURE 103 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 104 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 105 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 106 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.