Europe Sepsis Diagnostics Market Analysis and Insights
Sepsis is a potentially life-threatening condition that occurs when the body's response to an infection damages its own tissues. When the infection-fighting processes turn on the body, they cause organs to function poorly and abnormally. Sepsis may progress to septic shock. This is a dramatic drop in blood pressure that can lead to severe organ problems and death. To be diagnosed with sepsis, it is must to confirm the infection with signs such as change in mental status, systolic blood pressure, respiratory rate. Most often, sepsis occurs in people who are hospitalized or who have recently been hospitalized. People in an intensive care unit are more likely to develop infections that can then lead to sepsis. For the diagnosis of sepsis along with blood test other lab test needs to be done. Blood samples are used to test for evidence of infection, clotting problems, abnormal liver or kidney function, impaired oxygen availability, electrolyte imbalances. People who have sepsis require close monitoring and treatment in a hospital intensive care unit. Lifesaving measures may be needed to stabilize breathing and heart function.
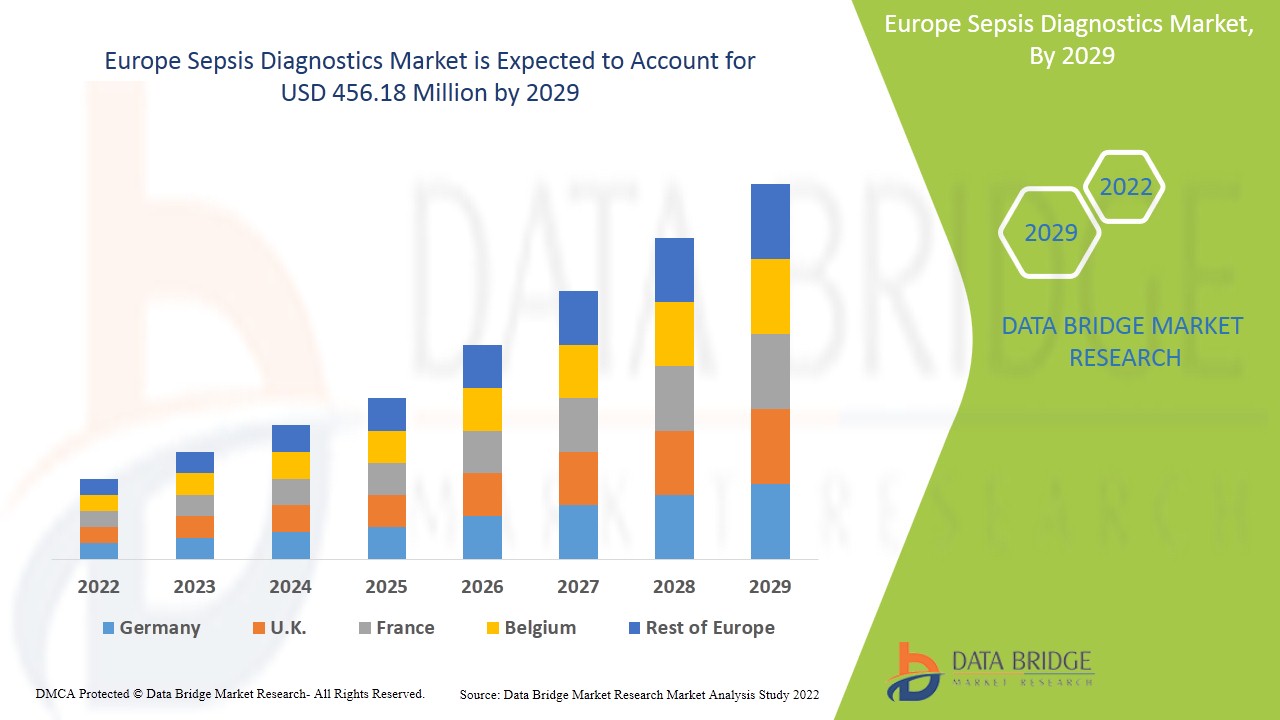
Europe Sepsis diagnostics market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 6.9% in the forecast period of 2022 to 2029 and is expected to reach USD 456.18 million by 2029 from USD 249.49 million in 2021.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2019-2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Techniques (Immunoassay, Molecular Diagnostic, Microbiology & Flow Cytometry), Test Type (Laboratory Testing & Point of Care Testing) |
|
Countries Covered |
Germany, France, U.K. and Rest of Europe in Europe |
|
Market Players Covered |
The major companies which are dealing in the market are Trinity Biotech (Ireland), Meridian Bioscience (U.S.), Omega Diagnostics Group PLC. (U.K.), Xcyton Diagnostics Limited (india), Diasorin S.p.A (Italy), Seegene Inc. (South Korea), EKF Diagnostics Holdings plc (U.K.), Axis-Shield Diagnostics Ltd. (U.K.), Immunexpress Inc. (U.S.), Luminex Corporation (U.S.), bioMérieux SA (France), BD (U.S.), Thermo Fisher Scientific Inc.(U.S.), Abbott (U.S), Roche Diagnostics (U.S), Cepheid (U.S.), Beckman Coulter, Inc. (U.S.), T2 Biosystems, Inc. (U.S.), Bruker (U.S.) and Ortho Clinical Diagnostics (U.S.). |
Sepsis Diagnostics Market Dynamics
Drivers
- Rising incidence of hospital-acquired infections
With the rapid increase in HAIs worldwide, there is an increase in the demand for proper sepsis diagnostic products will increase in upcoming years. Therefore, rising incidences of hospital-acquired infections are expected to act as a driver for the growth of the sepsis diagnostics market.
- Rising healthcare expenditure
Another significant factor influencing the growth rate of sepsis diagnostics market is the rising healthcare expenditure which helps in improving its infrastructure.
- Growing prevalence of sepsis
Growing prevalence of sepsis in various regions worldwide and various infections associated with it are expected to act as drivers for the growth of the sepsis diagnostics market
Furthermore, advancement in medical technology, rising initiatives by public and private organizations to spread awareness and growing government funding are the factors that will expand the sepsis diagnostics market.
Opportunities
- Evolution of novel biomarkers for sepsis diagnosis
Sepsis is one of the leading causes of mortality and morbidity, even with the current availability of extended-spectrum antibiotics and advanced medical care. Biomarkers offer a tool in facilitating early diagnosis, in identifying patient populations at high risk of complications, and in monitoring the progression of the disease, which are critical assessments for appropriate therapy and improvement in patient outcomes.
Also, the launch of effective therapies and continuous clinical trials will provide beneficial opportunities for the sepsis diagnostics market in the forecast period of 2022-2029. Also, high unmet need of current treatment and developments in healthcare technology will escalate the growth rate sepsis diagnostics market in future.
Restraints/Challenges
However, high cost of diagnosis and lack of appropriate testing for sepsis will impede the growth rate of sepsis diagnostics market. Additionally, lack of awareness about sepsis will further challenge the market in the forecast period mentioned above.
This sepsis diagnostics market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on sepsis diagnostics market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post COVID-19 Impact on Sepsis Diagnostics Market
The COVID-19 has negatively affected the market. Lockdowns and isolation during pandemics complicate the disease management and medication adherence. The lack of access to health-care facilities for routine treatment and medication administration will further affect the market. Social isolation increases stress, despair, and social support, all of which may cause a reduction in sepsis medication adherence during the pandemic.
Recent Development
- In July 2021, DiaSorin S.p.A announced that they acquired Luminex Corporation which develops, manufactures, and markets biological testing technologies in the clinical diagnostic and life science industries. This acquisition will help company gain technological advancements for its current product portfolio thereby expected in improving its position in market.
Europe Sepsis Diagnostics Market Scope
The Europe sepsis diagnostics market is segmented into techniques and test type. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Techniques
- Immunoassay
- Molecular Diagnostic
- Microbiology
- Flow Cytometry
On the basis of techniques, the Europe sepsis diagnostics market is segmented into microbiology, molecular diagnostic, immunoassay, and flow cytometry. The segment of immunoassay is further sub-segmented into Procalcitonin (PCT), Interleukin-6 (IL-6), C-Reactive Protein (CRP), Pentraxin-3 (PTX3), Calprotectin and Others.
Test Type
- Laboratory Testing
- Point of Care Testing
On the basis of test type, the Europe sepsis diagnostics market is segmented into laboratory testing and point of care testing.
Sepsis Diagnostics Market Regional Analysis/Insights
The sepsis diagnostics market is analysed and market size insights and trends are provided by country, techniques and test type as referenced above.
The countries covered in the Europe sepsis diagnostics market report are Germany, U.K., France and Rest of Europe.
Germany dominates the Europe sepsis diagnostics market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the fact that residents in lower socioeconomic status along with lower proximity of medical services in Germany are associated with their increased sepsis incidence. The rising number of market players and their manufacturing facilities in the country is further enhancing the market growth.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Sepsis Diagnostics Market Share Analysis
The sepsis diagnostics market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on sepsis diagnostics market.
Some of the major players operating in the sepsis diagnostics market are Trinity Biotech (Ireland), Meridian Bioscience, Omega Diagnostics Group PLC., Xcyton Diagnostics Limited, Diasorin S.p.A, Seegene Inc., EKF Diagnostics Holdings plc., Axis-Shield Diagnostics Ltd., Immunexpress Inc., Luminex Corporation, bioMérieux SA, BD, Thermo Fisher Scientific Inc. Abbott, Roche Diagnostics, Cepheid, Beckman Coulter, Inc., T2 Biosystems, Inc., and Bruker Ortho Clinical Diagnostics among others.
Research Methodology: Europe Sepsis Diagnostics Market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Global vs Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE SEPSIS DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TECHNIQUES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TECHNIQUES COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN INCIDENCE OF HOSPITAL-ACQUIRED INFECTIONS
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 GROW IN PREVALENCE OF SEPSIS
5.1.4 RISE IN TECHNOLOGICAL ADVANCEMENTS OF SEPSIS DIAGNOSTIC DEVICES
5.2 RESTRAINTS
5.2.1 HIGH COST OF DIAGNOSIS
5.2.2 LACK OF APPROPRIATE TESTING FOR SEPSIS
5.3 OPPORTUNITIES
5.3.1 DEVELOPMENT OF RAPID DIAGNOSTIC/POINT OF CARE (POC) TECHNIQUES FOR EARLY SEPSIS DIAGNOSIS
5.3.2 EVOLUTION OF NOVEL BIOMARKERS FOR SEPSIS DIAGNOSIS
5.4 CHALLENGES
5.4.1 LACK OF AWARENESS ABOUT SEPSIS
5.4.2 SHORTAGE OF SKILLED HEALTHCARE PROFESSIONALS
6 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES
6.1 OVERVIEW
6.2 IMMUNOASSAY
6.2.1 PROCALCITONIN (PCT)
6.2.2 INTERLEUKIN-6 (IL-6)
6.2.3 C-REACTIVE PROTEIN (CRP)
6.2.4 PENTRAXIN-3 (PTX3)
6.2.5 CALPROTECTIN
6.2.6 OTHERS
6.3 MOLECULAR DIAGNOSTIC
6.4 MICROBIOLOGY
6.5 FLOW CYTOMETRY
7 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 LABORATORY TESTING
7.3 POINT OF CARE TESTING
8 EUROPE SEPSIS DIAGNOSTICS MARKET, BY REGION
8.1 EUROPE
8.1.1 OVERVIEW
8.1.2 GERMANY
8.1.3 U.K.
8.1.4 FRANCE
8.1.5 REST OF EUROPE
9 EUROPE SEPSIS DIAGNOSTICS MARKET: COMPANY LANDSCAPE
9.1 COMPANY SHARE ANALYSIS: EUROPE
10 COMPANY PROFILE
10.1 ROCHE DIAGNOSTICS
10.1.1 COMPANY SNAPSHOT
10.1.2 REVENUE ANALYSIS
10.1.3 COMPANY SHARE ANALYSIS
10.1.4 PRODUCT PORTFOLIO
10.1.5 RECENT DEVELOPMENT
10.2 ABBOTT.
10.2.1 COMPANY SNAPSHOT
10.2.2 REVENUE ANALYSIS
10.2.3 COMPANY SHARE ANALYSIS
10.2.4 PRODUCT PORTFOLIO
10.2.5 RECENT DEVELOPMENT
10.2.5.1 PRODUCT LAUNCH
10.3 BIOMERIEUX SA
10.3.1 COMPANY SNAPSHOT
10.3.2 REVENUE ANALYSIS
10.3.3 COMPANY SHARE ANALYSIS
10.3.4 PRODUCT PORTFOLIO
10.3.5 RECENT DEVELOPMENT
10.3.5.1 PRODUCT LAUNCH
10.4 THERMO FISHER SCINETIFIC INC.
10.4.1 COMPANY SNAPSHOT
10.4.2 REVENUE ANALYSIS
10.4.3 COMPANY SHARE ANALYSIS
10.4.4 PRODUCT PORTFOLIO
10.4.5 RECENT DEVELOPMENT
10.4.5.1 Acquisition
10.5 BD
10.5.1 COMPANY SNAPSHOT
10.5.2 REVENUS ANALYSIS
10.5.3 COMPANY SHARE ANALYSIS
10.5.4 PRODUCT PORTFOLIO
10.5.5 RECENT DEVELOPMENT
10.6 SEEGENE INC.
10.6.1 COMPANY SNAPSHOT
10.6.2 REVENUE ANALYSIS
10.6.3 PRODUCT PORTFOLIO
10.6.4 RECENT DEVELOPMENT
10.7 AXIS-SHIELD DIAGNOSTICS LTD.
10.7.1 COMPANY SNAPSHOT
10.7.2 PRODUCT PORTFOLIO
10.7.3 RECENT DEVELOPMENTS
10.8 BECKMAN COULTER, INC.
10.8.1 COMPANY SNAPSHOT
10.8.2 PRODUCT PORTFOLIO
10.8.3 RECENT DEVELOPMENTS
10.9 BRUKER
10.9.1 COMPANY SNAPSHOT
10.9.2 REVENUS ANALYSIS
10.9.3 PRODUCT PORTFOLIO
10.9.4 RECENT DEVELOPMENTS
10.1 CEPHEID
10.10.1 COMPANY SNAPSHOT
10.10.2 REVENUE ANALYSIS
10.10.3 PRODUCT PORTFOLIO
10.10.4 RECENT DEVELOPMENT
10.10.4.1 Program launch
10.11 DIASORIN S.P.A.
10.11.1 COMPANY SNAPSHOT
10.11.2 REVENUE ANALYSIS
10.11.3 PRODUCT PORTFOLIO
10.11.4 RECENT DEVELOPMENT
10.11.4.1 ACQUISITION
10.12 EKF DIAGNOSTICS HOLDINGS PLC
10.12.1 COMPANY SNAPSHOT
10.12.2 REVENUE ANALYSIS
10.12.3 PRODUCT PORTFOLIO
10.12.4 RECENT DEVELOPMENT
10.12.4.1 Acquisition
10.13 IMMUNEXPRESS INC.
10.13.1 COMPANY SNAPSHOT
10.13.2 PRODUCT PORTFOLIO
10.13.3 RECENT DEVELOPMENT
10.13.3.1 PRODUCT LAUNCH
10.14 LUMINEX CORPORATION.
10.14.1 COMPANY SNAPSHOT
10.14.2 PRODUCT PORTFOLIO
10.14.3 RECENT DEVELOPMENT
10.14.3.1 Acquisition
10.15 MERIDIAN BIOSCIENCE
10.15.1 COMPANY SNAPSHOT
10.15.2 REVENUS ANALYSIS
10.15.3 PRODUCT PORTFOLIO
10.15.4 RECENT DEVELOPMENTS
10.16 OMEGA DIAGNOSTICS GROUP PLC
10.16.1 COMPANY SNAPSHOT
10.16.2 REVENUE ANALYSIS
10.16.3 PRODUCT PORTFOLIO
10.16.4 RECENT DEVELOPMENT
10.17 ORTHO CLINICAL DIAGNOSTICS.
10.17.1 COMPANY SNAPSHOT
10.17.2 REVENUE ANALYSIS
10.17.3 PRODUCT PORTFOLIO
10.17.4 RECENT DEVELOPMENT
10.17.4.1 PRODUCT LAUNCH
10.18 T2 BIOSYSTEM, INC.
10.18.1 COMPANY SNAPSHOT
10.18.2 PRODUCT PORTFOLIO
10.18.3 RECENT DEVELOPMENT
10.18.3.1 PRODUCT LAUNCH
10.19 TRINITY BIOTECH
10.19.1 COMPANY SNAPSHOT
10.19.2 REVENUE ANALYSIS
10.19.3 PRODUCT PORTFOLIO
10.19.4 RECENT DEVELOPMENT
10.2 XCTON DAGNOSTICS LIMITED
10.20.1 COMPANY SNAPSHOT
10.20.2 PRODUCT PORTFOLIO
10.20.3 RECENT DEVELOPMENT
11 QUESTIONNAIRE
12 RELATED REPORTS
List of Table
TABLE 1 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 2 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 3 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 4 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 5 EUROPE MOLECULAR DIAGNOSTICS IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 6 EUROPE MICROBIOLOGY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 7 EUROPE FLOW CYTOMETRY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 8 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 9 EUROPE LABORATORY TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 10 EUROPE POINT OF CARE TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 11 EUROPE SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 12 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 13 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 14 EUROPE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 15 EUROPE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 16 GERMANY SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 17 GERMANY IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 18 GERMANY IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 19 GERMANY SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 20 U.K. SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 21 U.K. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 22 U.K. IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 23 U.K. SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 24 FRANCE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 25 FRANCE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 26 FRANCE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 27 FRANCE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 28 REST OF EUROPE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
List of Figure
FIGURE 1 EUROPE SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 EUROPE SEPSIS DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE SEPSIS DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE SEPSIS DIAGNOSTICS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE SEPSIS DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE SEPSIS DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE SEPSIS DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE SEPSIS DIAGNOSTICS MARKET: MARKET TECHNIQUES COVERAGE GRID
FIGURE 9 EUROPE SEPSIS DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF SEPSIS IS EXPECTED TO DRIVE THE EUROPE SEPSIS DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 TECHNIQUES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE SEPSIS DIAGNOSTICS MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA TO DOMINATE AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE EUROPE SEPSIS DIAGNOSTICS MARKET FROM 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINT, OPPORTUNITIES, AND CHALLENGES FOR THE EUROPE SEPSIS DIAGNOSTICS MARKET
FIGURE 15 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2021
FIGURE 16 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2022-2029 (USD MILLION)
FIGURE 17 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, CAGR (2022-2029)
FIGURE 18 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, LIFELINE CURVE
FIGURE 19 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2021
FIGURE 20 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 21 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 22 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 23 EUROPE SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 24 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 25 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 26 EUROPE SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 27 EUROPE SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 28 EUROPE SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.