Europe Lymphangioleiomyomatosis Lam Market
Market Size in USD Million
CAGR :
% 
 USD
39.90 Million
USD
55.03 Million
2024
2032
USD
39.90 Million
USD
55.03 Million
2024
2032
| 2025 –2032 | |
| USD 39.90 Million | |
| USD 55.03 Million | |
|
|
|
|
Europe Lymphangioleiomyomatosis (LAM) Market Size
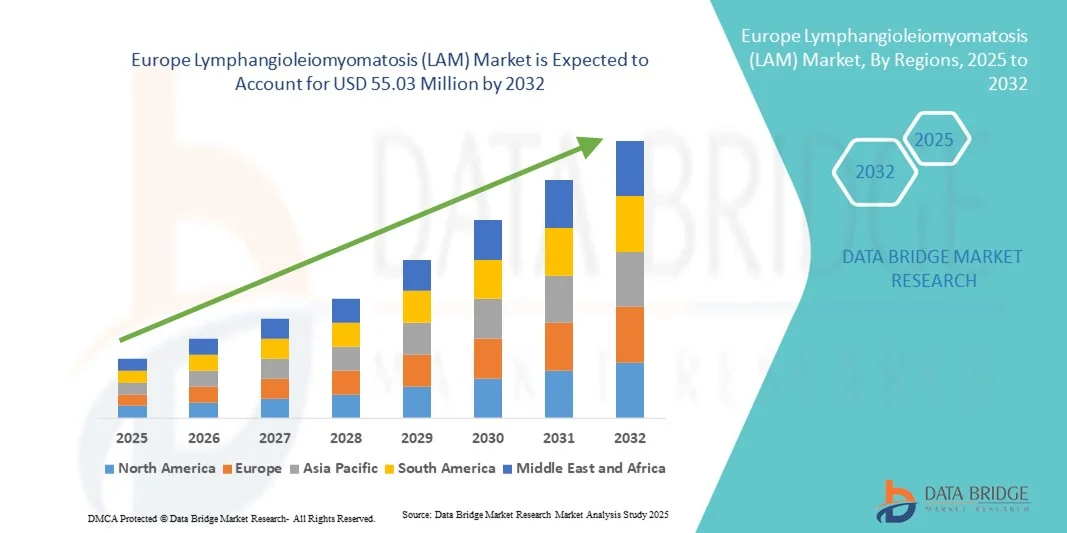
- The Europe Lymphangioleiomyomatosis (LAM) market size was valued at USD 39.90 million in 2024 and is expected to reach USD 55.03 million by 2032, at a CAGR of 4.10% during the forecast period
- The market growth is primarily driven by increasing awareness and diagnosis rates of rare lung disorders across European nations, coupled with advancements in precision medicine and molecular diagnostics
- In addition, growing research collaborations, improved access to orphan drugs, and expanding clinical trials for targeted therapies are enhancing treatment outcomes and patient management. These combined factors are accelerating the adoption of innovative LAM therapies, thereby strengthening the region’s overall market growth
Europe Lymphangioleiomyomatosis (LAM) Market Analysis
- Lymphangioleiomyomatosis (LAM), a rare progressive lung disease primarily affecting women, is gaining increasing focus across Europe due to improved clinical awareness, advancements in rare disease research, and enhanced diagnostic techniques involving high-resolution imaging and genetic testing
- The growing need for effective LAM therapies is driven by expanding access to sirolimus-based treatments, rising investments in rare disease programs, and collaborative research supported by academic institutions and pharmaceutical companies
- The United Kingdom dominated the Europe LAM market with the largest revenue share of 35.6% in 2024, supported by strong clinical infrastructure, active patient organizations such as LAM Action, and significant government backing for orphan drug development and rare disease registries
- Germany is expected to be the fastest-growing country in the Europe LAM market during the forecast period, fueled by increased clinical trial activity, advanced hospital networks specializing in pulmonary disorders, and expanding adoption of personalized treatment approaches
- The Treatment segment dominated the market with the largest market share of 62.3% in 2024, driven by the increasing adoption of therapies targeting LAM symptoms and disease progression, along with the rising availability of specialized care in hospitals and specialty clinics across Europe
Report Scope and Europe Lymphangioleiomyomatosis (LAM) Market Segmentation
|
Attributes |
Europe Lymphangioleiomyomatosis (LAM) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Europe Lymphangioleiomyomatosis (LAM) Market Trends
Rising Focus on Early Diagnosis and Rare Disease Awareness
- A significant and accelerating trend in the Europe LAM market is the growing emphasis on early diagnosis through high-resolution imaging, genetic testing, and patient registries, enabling timely interventions and improved patient outcomes
- For instance, national rare disease networks in the U.K. and France are implementing standardized diagnostic protocols for LAM, facilitating earlier detection and better management of disease progression
- Advances in molecular diagnostics and biomarker identification are allowing clinicians to differentiate between sporadic LAM and Tuberous Sclerosis Complex (TSC)-associated LAM, enhancing personalized treatment planning
- Specialized patient advocacy groups and awareness campaigns across Europe are educating both healthcare providers and patients about LAM symptoms, improving diagnosis rates and reducing delays in treatment initiation
- This trend towards more proactive screening, early detection, and disease awareness is reshaping expectations for patient care, with companies and research institutions increasingly focusing on diagnostic innovation
- The demand for early diagnostic solutions is growing rapidly across hospitals and specialty clinics, as clinicians and patients prioritize timely identification of LAM to improve long-term management and quality of life
- Increasing collaboration between pharmaceutical companies and research institutions is promoting the development of advanced diagnostic kits and predictive tools for LAM. Integration of digital health solutions and telemedicine in LAM patient management is enabling remote monitoring and faster diagnostic consultations, further accelerating early detection
Europe Lymphangioleiomyomatosis (LAM) Market Dynamics
Driver
Increasing Adoption of Targeted Therapies and Orphan Drugs
- The growing availability and adoption of targeted therapies, including sirolimus-based treatments, is a significant driver for the Europe LAM market, improving patient outcomes and stabilizing lung function
- For instance, in March 2024, the U.K.’s National Health Service expanded access to sirolimus therapy for sporadic LAM patients, supporting broader adoption of effective treatment options
- Rising investments in rare disease research and orphan drug programs by both public institutions and pharmaceutical companies are accelerating the development of new LAM therapies
- Increasing awareness among clinicians regarding mTOR inhibitors and other targeted treatments is leading to more patients receiving guideline-recommended therapy, boosting market growth
- Enhanced patient monitoring through specialized clinics, registries, and telemedicine support is facilitating adherence to therapy and long-term disease management
- Growing recognition of the benefits of targeted therapy and the expansion of specialized care networks across Europe are driving higher adoption of LAM treatments in hospitals and specialty clinics
- Expansion of clinical trials and real-world evidence studies across Germany, France, and Italy is supporting new therapy approvals and increasing confidence in treatment efficacy. Partnerships between pharmaceutical companies and rare disease advocacy groups are improving patient access programs and education, further propelling treatment uptake
Restraint/Challenge
Limited Awareness and High Treatment Costs
- Limited awareness of LAM among general practitioners and some pulmonologists remains a key challenge, often resulting in delayed diagnosis and treatment initiation, which can hinder market growth
- For instance, surveys in Germany and Italy indicate that many patients experience a diagnostic delay of several years due to lack of familiarity with rare lung diseases among frontline clinicians
- The high cost of sirolimus and other orphan therapies can restrict access, particularly in countries with budget constraints or limited insurance coverage for rare diseases
- While patient support programs and government subsidies are improving accessibility, the financial burden of treatment remains a barrier for some patients and healthcare provider
- Ensuring wider awareness through educational campaigns, clinical guidelines, and physician training is crucial to improve early diagnosis and treatment uptake
- Overcoming high treatment costs through reimbursement initiatives, patient assistance programs, and policy support is necessary to facilitate broader access and strengthen market growth
- Variability in healthcare infrastructure across European countries can create inconsistent access to specialized LAM care and diagnostics, limiting market penetration. Regulatory challenges related to orphan drug approvals and reimbursement procedures can delay therapy availability, constraining market expansion
Europe Lymphangioleiomyomatosis (LAM) Market Scope
The market is segmented on the basis of disease type, type, complications, route of administration, end user, and distribution channel.
- By Disease Type
On the basis of disease type, the Europe LAM market is segmented into tuberous sclerosis complex LAM (TSC-LAM), sporadic LAM, and others. The Sporadic LAM segment dominated the market with the largest revenue share of 58.4% in 2024, due to its higher prevalence among women of reproductive age. Sporadic LAM patients require continuous monitoring and targeted therapy, which sustains treatment demand. Extensive clinical research, established treatment guidelines, and active patient registries across Europe facilitate early diagnosis and intervention. Hospitals and specialty clinics increasingly focus on Sporadic LAM because of its progressive nature and need for mTOR inhibitor therapy. European countries such as the U.K., Germany, and France are leading in treatment adoption and patient management. Multidisciplinary care involving pulmonologists, nephrologists, and genetic specialists further reinforces the segment’s dominance.
The Tuberous Sclerosis Complex LAM (TSC-LAM) segment is expected to witness the fastest CAGR of 7.9% from 2025 to 2032, fueled by rising awareness of TSC among genetic counselors and pulmonologists. TSC-LAM patients require multidisciplinary care including nephrology and pulmonology consultations. Improved genetic testing and early identification programs facilitate timely treatment initiation. The development of TSC-focused clinical guidelines and European rare disease networks supports growth. Patient advocacy and educational campaigns are increasing diagnosis rates, contributing to market expansion. Ongoing research collaborations for TSC-LAM treatments are attracting investments in specialized therapies.
- By Type
On the basis of type, the market is segmented into diagnosis and treatment. The Treatment segment dominated with a revenue share of 62.3% in 2024, driven by the increasing adoption of mTOR inhibitors such as sirolimus for stabilizing lung function. Hospitals and specialty clinics are the primary channels for treatment administration, and growing clinical trial activity drives adoption. Rising awareness about disease progression and the availability of approved orphan drugs reinforce demand. Treatments for LAM are increasingly integrated with patient monitoring programs to ensure adherence. Europe’s healthcare infrastructure, particularly in Western Europe, facilitates access to advanced therapies. Long-term therapy for chronic management also contributes to revenue growth in this segment.
The Diagnosis segment is expected to witness the fastest growth at 8.1% CAGR from 2025 to 2032, driven by advancements in high-resolution CT imaging, pulmonary function testing, and biomarker identification. Early diagnosis is critical in LAM to prevent irreversible lung damage. Hospitals and diagnostic centers are adopting genetic testing for TSC-LAM differentiation. European rare disease initiatives promote diagnostic awareness and physician training programs. Telemedicine and digital reporting enhance accessibility of LAM diagnostics. Rising patient advocacy and disease awareness campaigns support faster adoption. Increased funding for rare disease diagnostic programs is also contributing to segment growth.
- By Complications
On the basis of complications, the market is segmented into pneumothorax, chylothorax, kidney tumor, pleural effusions, swelling & fluid build-up, and others. The Pneumothorax segment dominated with a share of 41.7% in 2024, as recurrent lung collapse is a severe complication requiring hospitalization and intervention. Management of pneumothorax often involves multidisciplinary care, contributing to higher treatment costs. Specialty clinics and hospitals provide monitoring and interventional procedures. Patient awareness and early detection of pneumothorax risk in LAM are increasing across Europe. Standardized clinical guidelines for complication management support the segment’s dominance. Hospitals in Germany, France, and the U.K. are leading in advanced care and intervention for pneumothorax.
The Chylothorax segment is expected to witness the fastest growth at 9.2% CAGR from 2025 to 2032, fueled by rising prevalence of lymphatic involvement in LAM patients. European centers of excellence for rare lung diseases are expanding services for chylothorax management. Early diagnosis and minimally invasive interventions are driving adoption. Awareness campaigns highlighting this complication are increasing patient engagement. Specialty clinics and hospitals are investing in advanced treatment techniques. Clinical research focusing on lymphatic complications supports segment growth.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral, parenteral, and others. The Oral segment dominated with a revenue share of 69.1% in 2024, driven by widespread use of oral sirolimus therapy for long-term management. Oral administration is preferred due to convenience, patient adherence, and outpatient suitability. Hospitals, specialty clinics, and home healthcare services facilitate oral therapy management. European reimbursement policies and patient assistance programs support adoption. Oral administration integrates easily with monitoring programs for dose adjustments. Physicians often prefer oral therapy for its ease of use in chronic care plans.
The Parenteral segment is expected to witness the fastest growth at 8.5% CAGR from 2025 to 2032, fueled by injectable therapies for advanced cases or oral-intolerant patients. Parenteral administration is typically used in hospital settings under specialist supervision. Innovations in biologics and targeted therapies contribute to growth. Clinical trials in Germany, France, and Italy support market expansion. Hospital pharmacies and specialty clinics are increasingly offering parenteral administration. Telemedicine and patient support programs facilitate therapy adherence.
- By End User
On the basis of end user, the market is segmented into hospitals, specialty clinics, diagnostic centers, home healthcare, and others. The Hospitals segment dominated with a market share of 53.4% in 2024, driven by concentration of specialized LAM treatment and monitoring facilities. Hospitals provide integrated care including diagnostics, treatment, and complication management. They serve as primary centers for clinical trials and rare disease research. European hospitals increasingly collaborate with patient advocacy groups for education and adherence programs. Advanced infrastructure and multidisciplinary care models support this segment’s dominance. Hospitals remain the preferred choice for severe or complex LAM cases.
The Specialty Clinics segment is expected to witness the fastest growth at 9.0% CAGR from 2025 to 2032, supported by rising numbers of centers focused on rare lung disorders. Specialty clinics offer targeted care for LAM patients, often providing long-term monitoring and personalized therapy. Adoption of digital health solutions and telemedicine enhances service reach. Clinics are integrating multidisciplinary approaches including nephrology and genetics consultations for TSC-LAM cases. Patient awareness initiatives are driving demand for specialty clinic services. Collaboration with hospitals and rare disease networks strengthens clinic capabilities and patient outcomes.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into direct tender, hospital pharmacies, retail pharmacies, online pharmacies, and others. The Hospital Pharmacies segment dominated with a revenue share of 47.8% in 2024, as hospitals remain the primary point for dispensing mTOR inhibitors and other LAM therapies. Hospital pharmacies ensure proper storage, adherence monitoring, and patient counseling. They are closely linked with clinical trials and treatment programs. European hospital pharmacies are expanding capacity to meet rising patient demand. They also provide education on drug administration and side effect management. Integration with specialty clinics enhances distribution efficiency and patient compliance.
The Online Pharmacies segment is expected to witness the fastest growth at 10.3% CAGR from 2025 to 2032, fueled by increasing digital adoption, home delivery services, and improved access to rare disease medications. Online platforms provide convenience for patients in remote areas. Telemedicine integration with online pharmacies enhances adherence and follow-up. Patient education initiatives and direct-to-patient delivery models further drive adoption. Growing awareness of rare disease treatments encourages online procurement. Digital prescription management systems support safe and timely delivery of medications.
Europe Lymphangioleiomyomatosis (LAM) Market Regional Analysis
- The United Kingdom dominated the Europe LAM market with the largest revenue share of 35.6% in 2024, supported by strong clinical infrastructure, active patient organizations such as LAM Action, and significant government backing for orphan drug development and rare disease registries
- Patients and clinicians in the U.K. benefit from advanced diagnostic facilities, specialized treatment centers, and access to orphan drugs, which together improve disease management and outcomes for LAM patients
- This widespread adoption is further supported by high awareness among pulmonologists, growing clinical trial activity, and national rare disease initiatives, establishing the U.K. as a key hub for LAM diagnosis and treatment in Europe
U.K. Lymphangioleiomyomatosis (LAM) Market Insight
The United Kingdom dominated the Europe LAM market with the largest revenue share of 35.6% in 2024, driven by well-established healthcare infrastructure, strong government support for rare disease programs, and active patient advocacy networks such as LAM Action. Hospitals and specialty clinics in the U.K. provide advanced diagnostic facilities, including high-resolution imaging and genetic testing, enabling early detection and effective disease management. The widespread availability of sirolimus therapy and other targeted treatments contributes to the high adoption rate. National rare disease initiatives and registry programs facilitate timely diagnosis and long-term patient monitoring.
Germany Lymphangioleiomyomatosis (LAM) Market Insight
The Germany LAM market is expected to expand at a considerable CAGR, fueled by growing awareness of rare pulmonary disorders, advanced healthcare infrastructure, and increasing clinical trial activity. German hospitals and specialty clinics provide comprehensive diagnostic and treatment services, supporting patient care for Sporadic and TSC-LAM. Adoption of mTOR inhibitors and multidisciplinary management involving pulmonology, nephrology, and genetics is driving market growth. Research initiatives and government support for rare disease programs enhance therapy access. Patient education and registry-based monitoring facilitate early diagnosis and treatment adherence. The market benefits from strong reimbursement policies and well-established rare disease networks.
France Lymphangioleiomyomatosis (LAM) Market Insight
The France LAM market is witnessing growth due to the presence of specialized LAM centers, access to advanced therapies, and growing patient awareness. Hospitals and diagnostic centers are integrating high-resolution imaging and genetic testing for early detection of LAM. Collaborative research and European Reference Networks for rare lung diseases improve patient outcomes. Government support for rare disease policy and reimbursement facilitates access to orphan drugs. Clinical trials and patient support programs enhance treatment adoption. Awareness campaigns targeting clinicians and patients improve early intervention rates and overall market expansion.
Italy Lymphangioleiomyomatosis (LAM) Market Insight
The Italy LAM market is expected to grow steadily, supported by expanding healthcare infrastructure, patient registries, and increasing access to orphan drugs. Hospitals and specialty clinics focus on early diagnosis and long-term management of LAM patients. Targeted therapies, especially sirolimus, are increasingly adopted due to proven efficacy in stabilizing lung function. Italian centers actively participate in rare disease research networks to promote treatment accessibility. Awareness campaigns and training for pulmonologists reduce diagnostic delays. Telemedicine and digital patient monitoring enhance adherence and follow-up care.
Europe Lymphangioleiomyomatosis (LAM) Market Share
The Europe Lymphangioleiomyomatosis (LAM) industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- Johnson & Johnson Services, Inc. (U.S.)
- GSK plc (U.K.)
- AstraZeneca (U.K.)
- Dr. Reddy’s Laboratories Ltd (India)
- Zydus Pharmaceuticals, Inc. (India)
- Intas Pharmaceuticals Ltd (India)
- Apotex Inc. (Canada)
- Amneal Pharmaceuticals LLC (U.S.)
- Terumo Corporation (Japan)
- Hikma Pharmaceuticals plc (U.K.)
- TransMedics, Inc. (U.S.)
- XVIVO Perfusion AB (Sweden)
- Taj Pharmaceuticals Limited (India)
- Morgan Scientific Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd (India)
- Sanofi (France)
- Boehringer Ingelheim International GmbH (Germany)
What are the Recent Developments in Europe Lymphangioleiomyomatosis (LAM) Market?
- In March 2025, The LAM Foundation highlighted a new research project: “Targeting Fibroblast‑Endothelial Interactions in LAM Pathogenesis using 3D Spheroid Models and Spatial Transcriptomics”, signalling growing European collaboration in advanced molecular research and translational models aimed at novel therapeutic approaches in LAM
- In January 2025, a study published in Frontiers in Medicine titled “Characteristics and outcomes of patients with LAM” analysed a European patient cohort and provided updated real‑world data on disease progression, treatment uptake and outcomes, reinforcing the need for improved monitoring and specialized centres in Europe
- In April 2024, research published in European Radiology demonstrated that an ultra‑low‑dose chest CT protocol using a silver filter and deep‑learning reconstruction can reduce radiation dose by ~85% while maintaining diagnostic accuracy for LAM cyst scoring an important development for safer routine monitoring in Europe
- In February 2024, the Lymphangiomatosis & Gorham’s Disease Alliance published a report entitled “Navigating the Complex World of Lymphatic Anomalies: Breakthroughs from Lung Disease Research”, which highlighted new insights into LAM’s link to lymphatic disorders, mapping genetic mutations in the TSC2 gene and outlining how LAM research in Europe now offers broader implications for complex lymphatic anomalies
- In May 2021, a long‑term outcome study from Spain reported in Scientific Reports revealed that more than half of the LAM patients treated with Sirolimus sustained a positive response over five years, marking one of the largest European real‑world follow‑ups and supporting continued use of mTOR‑inhibitor therapy in LAM management
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.