Market Analysis and Insights: Asia-Pacific Sepsis Diagnostics Market
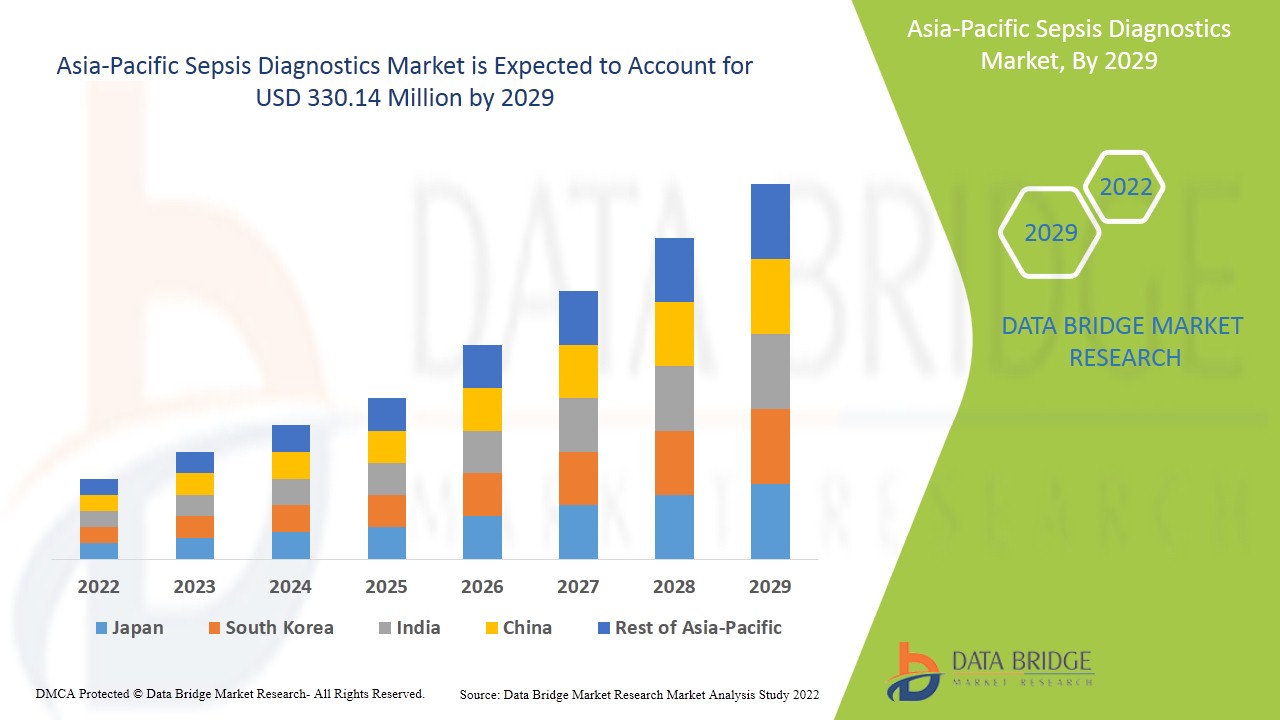
Sepsis diagnostics market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 8.9% in the forecast period of 2022 to 2029 and is expected to reach USD 330.14 million by 2029 from USD 156.07 million in 2021. Rising incidences of infections acquired in hospitals and increasing prevalence of sepsis which propelled the demand of the market in the forecast period.
Sepsis dates back over 2,700 years ago when it was first mentioned (medically) in the ancient Greek poems of Homer. In his poems he uses the word sepsis derived from the Greek word “sepo”, which means “I rot.” The term Sepsis was used in the writings of Hippocrates as well. He viewed sepsis as a dangerous biological decay that could potentially occur in the body. The Romans further developed theories on sepsis. One of the most well-known theories on sepsis came from the Roman physician Galen, whose techniques for wound healing lasted 1500 years. He believed that sepsis came from the production of invisible creatures that gave off fumes called “miasma.”
Ignaz Semmelweiss, a physician from Vienna, contributed to a significant discovery about sepsis in the 1800s. He worked on a maternity ward where he noticed that there was a high rate of death from childbed fever, also known as puerperal sepsis. After he found out that some of colleagues died due to the infections acquired from the surgeries which they have done which is autopsy. Semmelweiss concluded that there was a connection between puerperal sepsis and the doctors who performed autopsies. In the 60s, the first septic shock management strategy was developed by Edward Frank, who was an American physician. In 1991, the first sepsis conference was held to address the lack of consensus regarding the definition of sepsis, which created difficulty in sepsis diagnoses and treatment. 10 years later, in 2001, several North American and European societies convened a second sepsis conference to further define the diagnostic criteria of severe sepsis and septic shock and to develop management guidelines.
The increasing healthcare expenditure is expected to act as driver for the growth of the market. The lack of awareness about sepsis and its symptoms is expected to act as challenge for the growth of the market. The rising technological advancement leading to development of rapid diagnostics tests for early diagnosis of sepsis is expected to act as opportunity for the growth of the market. However, the shortage of healthcare professionals expected to challenge the market growth.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2019-2020 |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units |
|
Segments Covered |
By Techniques (Microbiology, Molecular Diagnostic, Immunoassay and Flow Cytometry), Test Type (Laboratory Testing and Point of Care Testing) |
|
Countries Covered |
China, India, Japan, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific |
|
Market Players Covered |
Trinity Biotech (Ireland), Meridian Bioscience, Omega Diagnostics Group PLC., Xcyton Diagnostics Limited, Diasorin S.p.A, Seegene Inc., EKF Diagnostics Holdings plc., Axis-Shield Diagnostics Ltd., Immunexpress Inc., Luminex Corporation, bioMérieux SA, BD, Thermo Fisher Scientific Inc. ,Abbott, Roche Diagnostics, Cepheid, Beckman Coulter, Inc., T2 Biosystems, Inc., and Bruker Ortho Clinical Diagnostics |
Sepsis Diagnostics Market Dynamics
Drivers
- Rise in incidence of hospital-acquired infections
The most common hospital-acquired infections (HAIs) are urinary tract infections, pneumonia, and sepsis. The rise in the incidences of hospital-acquired infections (HAIs) will increase the risk of sepsis and hence act as a major driver that will result in the expansion of the growth rate of the treatment market.
- Rise in healthcare expenditure
Another significant factor influencing the growth rate of sepsis diagnostics market is the rising healthcare expenditure which helps in improving its infrastructure.
- Grow in prevalence of sepsis
The rise in the prevalence of sepsis will further enhance the growth and hence act as a major driver for sepsis diagnostics market.
Furthermore, rise in technological advancements of sepsis diagnostic devices will expand the sepsis diagnostics market.
Opportunities
- Development of rapid diagnostic/point of care (POC) techniques for early sepsis diagnosis
Increasing need for the rapid diagnosis of sepsis to reduce the delay of antibiotic therapy among patients with sepsis will boost new opportunities for the market's growth rate.
Also, evolution of novel biomarkers for sepsis diagnosis will provide beneficial opportunities for the sepsis diagnostics market in the forecast period of 2022-2029.
Restraints/Challenges
However, lack of awareness about sepsis and shortage of skilled healthcare professionals will slow down the growth of sepsis diagnostics market. Additionally, high cost of diagnosis and lack of appropriate testing for sepsis will also prove to be harmful for a healthy growth of sepsis diagnostics market in the forecast period mentioned above.
This sepsis diagnostics market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on sepsis diagnostics market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post COVID-19 Impact on Sepsis Diagnostics Market
The COVID-19 has negatively affected the market. Lockdowns and isolation during pandemics complicate the disease management and medication adherence. The lack of access to health-care facilities for routine diagnosis/blood check-up, consultancy and medication administration will further affect the market. Social isolation increases dismissal of signs and symptoms of sepsis due to fear associated with visiting crowded hospitals and spread of misinformation during the pandemic.
Recent Development
- In April 2109, Bruker announced the portfolio expansion for Microbial Identification, Infection Control and Molecular Diagnostics of Infectious Diseases. This includes new assays, software and library extensions for its MALDI Biotyper, IR Biotyper and Fluorocycler XT product lines. This has helped company to increase its revenue through new expansions.
Asia-Pacific Sepsis Diagnostics Market Scope
The sepsis diagnostics market is segmented into techniques and test type. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Techniques
- Microbiology
- Molecular Diagnostic
- Immunoassay
- Flow Cytometry
On the basis of techniques, the Asia-Pacific sepsis diagnostics market is segmented into microbiology, molecular diagnostic, immunoassay, and flow cytometry.
Test Type
- Laboratory Testing
- Point of Care Testing
On the basis of test type, the Asia-Pacific sepsis diagnostics market is segmented into laboratory testing and point of care testing.
Sepsis Diagnostics Market Regional Analysis/Insights
The sepsis diagnostics market is analysed and market size insights and trends are provided by country, techniques and test type as referenced above.
The countries covered in the sepsis diagnostics market report are China, India, Japan, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, and rest of Asia-Pacific.
China is expected to dominate the Asia-Pacific sepsis diagnostics market due to higher availability of advanced healthcare system and rising number of hospitals, diagnostic centres and clinics which is driving the growth. Asia-Pacific sepsis diagnostics market is expected to grow due to increasing healthcare expenditure by government of developing nations.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of Asia-Pacific brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Sepsis Diagnostics Market Share Analysis
The sepsis diagnostics market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on sepsis diagnostics market.
Some of the major players operating in the sepsis diagnostics market are Trinity Biotech (Ireland), Meridian Bioscience, Omega Diagnostics Group PLC., Xcyton Diagnostics Limited, Diasorin S.p.A, Seegene Inc., EKF Diagnostics Holdings plc., Axis-Shield Diagnostics Ltd., Immunexpress Inc., Luminex Corporation, bioMérieux SA, BD, Thermo Fisher Scientific Inc., Abbott, Roche Diagnostics, Cepheid, Beckman Coulter, Inc., T2 Biosystems, Inc., and Bruker Ortho Clinical Diagnostics among others.
Research Methodology: Asia-Pacific Sepsis Diagnostics Market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Asia-Pacific vs Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TECHNIQUES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TECHNIQUES COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN INCIDENCE OF HOSPITAL-ACQUIRED INFECTIONS
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 GROW IN PREVALENCE OF SEPSIS
5.1.4 RISE IN TECHNOLOGICAL ADVANCEMENTS OF SEPSIS DIAGNOSTIC DEVICES
5.2 RESTRAINTS
5.2.1 HIGH COST OF DIAGNOSIS
5.2.2 LACK OF APPROPRIATE TESTING FOR SEPSIS
5.3 OPPORTUNITIES
5.3.1 DEVELOPMENT OF RAPID DIAGNOSTIC/POINT OF CARE (POC) TECHNIQUES FOR EARLY SEPSIS DIAGNOSIS
5.3.2 EVOLUTION OF NOVEL BIOMARKERS FOR SEPSIS DIAGNOSIS
5.4 CHALLENGES
5.4.1 LACK OF AWARENESS ABOUT SEPSIS
5.4.2 SHORTAGE OF SKILLED HEALTHCARE PROFESSIONALS
6 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES
6.1 OVERVIEW
6.2 IMMUNOASSAY
6.2.1 PROCALCITONIN (PCT)
6.2.2 INTERLEUKIN-6 (IL-6)
6.2.3 C-REACTIVE PROTEIN (CRP)
6.2.4 PENTRAXIN-3 (PTX3)
6.2.5 CALPROTECTIN
6.2.6 OTHERS
6.3 MOLECULAR DIAGNOSTIC
6.4 MICROBIOLOGY
6.5 FLOW CYTOMETRY
7 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 LABORATORY TESTING
7.3 POINT OF CARE TESTING
8 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET, BY REGION
8.1 ASIA-PACIFIC
8.1.1 OVERVIEW
8.1.2 CHINA
8.1.3 INDIA
8.1.4 JAPAN
8.1.5 SOUTH KOREA
8.1.6 AUSTRALIA
8.1.7 SINGAPORE
8.1.8 THAILAND
8.1.9 MALAYSIA
8.1.10 INDONESIA
8.1.11 PHILIPPINES
8.1.12 REST OF ASIA-PACIFIC
9 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: COMPANY LANDSCAPE
9.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
10 COMPANY PROFILE
10.1 ROCHE DIAGNOSTICS
10.1.1 COMPANY SNAPSHOT
10.1.2 REVENUE ANALYSIS
10.1.3 COMPANY SHARE ANALYSIS
10.1.4 PRODUCT PORTFOLIO
10.1.5 RECENT DEVELOPMENT
10.2 ABBOTT.
10.2.1 COMPANY SNAPSHOT
10.2.2 REVENUE ANALYSIS
10.2.3 COMPANY SHARE ANALYSIS
10.2.4 PRODUCT PORTFOLIO
10.2.5 RECENT DEVELOPMENT
10.2.5.1 PRODUCT LAUNCH
10.3 BIOMERIEUX SA
10.3.1 COMPANY SNAPSHOT
10.3.2 REVENUE ANALYSIS
10.3.3 COMPANY SHARE ANALYSIS
10.3.4 PRODUCT PORTFOLIO
10.3.5 RECENT DEVELOPMENT
10.3.5.1 PRODUCT LAUNCH
10.4 THERMO FISHER SCINETIFIC INC.
10.4.1 COMPANY SNAPSHOT
10.4.2 REVENUE ANALYSIS
10.4.3 COMPANY SHARE ANALYSIS
10.4.4 PRODUCT PORTFOLIO
10.4.5 RECENT DEVELOPMENT
10.4.5.1 Acquisition
10.5 BD
10.5.1 COMPANY SNAPSHOT
10.5.2 REVENUS ANALYSIS
10.5.3 COMPANY SHARE ANALYSIS
10.5.4 PRODUCT PORTFOLIO
10.5.5 RECENT DEVELOPMENT
10.6 SEEGENE INC.
10.6.1 COMPANY SNAPSHOT
10.6.2 REVENUE ANALYSIS
10.6.3 PRODUCT PORTFOLIO
10.6.4 RECENT DEVELOPMENT
10.7 AXIS-SHIELD DIAGNOSTICS LTD.
10.7.1 COMPANY SNAPSHOT
10.7.2 PRODUCT PORTFOLIO
10.7.3 RECENT DEVELOPMENTS
10.8 BECKMAN COULTER, INC.
10.8.1 COMPANY SNAPSHOT
10.8.2 PRODUCT PORTFOLIO
10.8.3 RECENT DEVELOPMENTS
10.9 BRUKER
10.9.1 COMPANY SNAPSHOT
10.9.2 REVENUS ANALYSIS
10.9.3 PRODUCT PORTFOLIO
10.9.4 RECENT DEVELOPMENTS
10.1 CEPHEID
10.10.1 COMPANY SNAPSHOT
10.10.2 REVENUE ANALYSIS
10.10.3 PRODUCT PORTFOLIO
10.10.4 RECENT DEVELOPMENT
10.10.4.1 Program launch
10.11 DIASORIN S.P.A.
10.11.1 COMPANY SNAPSHOT
10.11.2 REVENUE ANALYSIS
10.11.3 PRODUCT PORTFOLIO
10.11.4 RECENT DEVELOPMENT
10.11.4.1 ACQUISITION
10.12 EKF DIAGNOSTICS HOLDINGS PLC
10.12.1 COMPANY SNAPSHOT
10.12.2 REVENUE ANALYSIS
10.12.3 PRODUCT PORTFOLIO
10.12.4 RECENT DEVELOPMENT
10.12.4.1 Acquisition
10.13 IMMUNEXPRESS INC.
10.13.1 COMPANY SNAPSHOT
10.13.2 PRODUCT PORTFOLIO
10.13.3 RECENT DEVELOPMENT
10.13.3.1 PRODUCT LAUNCH
10.14 LUMINEX CORPORATION.
10.14.1 COMPANY SNAPSHOT
10.14.2 PRODUCT PORTFOLIO
10.14.3 RECENT DEVELOPMENT
10.14.3.1 Acquisition
10.15 MERIDIAN BIOSCIENCE
10.15.1 COMPANY SNAPSHOT
10.15.2 REVENUS ANALYSIS
10.15.3 PRODUCT PORTFOLIO
10.15.4 RECENT DEVELOPMENTS
10.16 OMEGA DIAGNOSTICS GROUP PLC
10.16.1 COMPANY SNAPSHOT
10.16.2 REVENUE ANALYSIS
10.16.3 PRODUCT PORTFOLIO
10.16.4 RECENT DEVELOPMENT
10.17 ORTHO CLINICAL DIAGNOSTICS.
10.17.1 COMPANY SNAPSHOT
10.17.2 REVENUE ANALYSIS
10.17.3 PRODUCT PORTFOLIO
10.17.4 RECENT DEVELOPMENT
10.17.4.1 PRODUCT LAUNCH
10.18 T2 BIOSYSTEM, INC.
10.18.1 COMPANY SNAPSHOT
10.18.2 PRODUCT PORTFOLIO
10.18.3 RECENT DEVELOPMENT
10.18.3.1 PRODUCT LAUNCH
10.19 TRINITY BIOTECH
10.19.1 COMPANY SNAPSHOT
10.19.2 REVENUE ANALYSIS
10.19.3 PRODUCT PORTFOLIO
10.19.4 RECENT DEVELOPMENT
10.2 XCTON DAGNOSTICS LIMITED
10.20.1 COMPANY SNAPSHOT
10.20.2 PRODUCT PORTFOLIO
10.20.3 RECENT DEVELOPMENT
11 QUESTIONNAIRE
12 RELATED REPORTS
List of Table
TABLE 1 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 2 ASIA PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 3 ASIA PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 4 ASIA PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 5 ASIA PACIFIC MOLECULAR DIAGNOSTICS IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 6 ASIA PACIFIC MICROBIOLOGY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 7 ASIA PACIFIC FLOW CYTOMETRY IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 8 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 9 ASIA PACIFIC LABORATORY TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 10 ASIA PACIFIC POINT OF CARE TESTING IN SEPSIS DIAGNOSTICS MARKET, BY REGION, 2019-2029 (USD MILLION)
TABLE 11 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY COUNTRY, 2019-2029 (USD MILLION)
TABLE 12 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 13 ASIA-PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 14 ASIA-PACIFIC IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 15 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 16 CHINA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 17 CHINA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 18 CHINA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 19 CHINA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 20 INDIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 21 INDIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 22 INDIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 23 INDIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 24 JAPAN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 25 JAPAN IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 26 JAPAN IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 27 JAPAN SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 28 SOUTH KOREA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 29 SOUTH KOREA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 30 SOUTH KOREA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 31 SOUTH KOREA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 32 AUSTRALIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 33 AUSTRALIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 34 AUSTRALIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 35 AUSTRALIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 36 SINGAPORE SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 37 SINGAPORE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 38 SINGAPORE IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 39 SINGAPORE SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 40 THAILAND SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 41 THAILAND IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 42 THAILAND IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 43 THAILAND SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 44 MALAYSIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 45 MALAYSIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 46 MALAYSIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 47 MALAYSIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 48 INDONESIA SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 49 INDONESIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 50 INDONESIA IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 51 INDONESIA SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 52 PHILIPPINES SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 53 PHILIPPINES IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
TABLE 54 PHILIPPINES IMMUNOASSAY IN SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (UNITS)
TABLE 55 PHILIPPINES SEPSIS DIAGNOSTICS MARKET, BY TEST TYPE, 2019-2029 (USD MILLION)
TABLE 56 REST OF ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET, BY TECHNIQUES, 2019-2029 (USD MILLION)
List of Figure
FIGURE 1 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: MARKET TECHNIQUES COVERAGE GRID
FIGURE 9 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF SEPSIS IS EXPECTED TO DRIVE THE ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 TECHNIQUES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA TO DOMINATE AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET FROM 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINT, OPPORTUNITIES, AND CHALLENGES FOR THE ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET
FIGURE 15 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2021
FIGURE 16 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, 2022-2029 (USD MILLION)
FIGURE 17 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, CAGR (2022-2029)
FIGURE 18 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES, LIFELINE CURVE
FIGURE 19 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2021
FIGURE 20 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 21 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 22 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 23 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: SNAPSHOT (2021)
FIGURE 24 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021)
FIGURE 25 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 26 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 27 ASIA-PACIFIC SEPSIS DIAGNOSTICS MARKET: BY TECHNIQUES (2022-2029)
FIGURE 28 ASIA PACIFIC SEPSIS DIAGNOSTICS MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.