Asia Pacific Preclinical Imaging Market
Market Size in USD Million
CAGR :
% 
 USD
294.06 Million
USD
451.29 Million
2024
2032
USD
294.06 Million
USD
451.29 Million
2024
2032
| 2025 –2032 | |
| USD 294.06 Million | |
| USD 451.29 Million | |
|
|
|
|
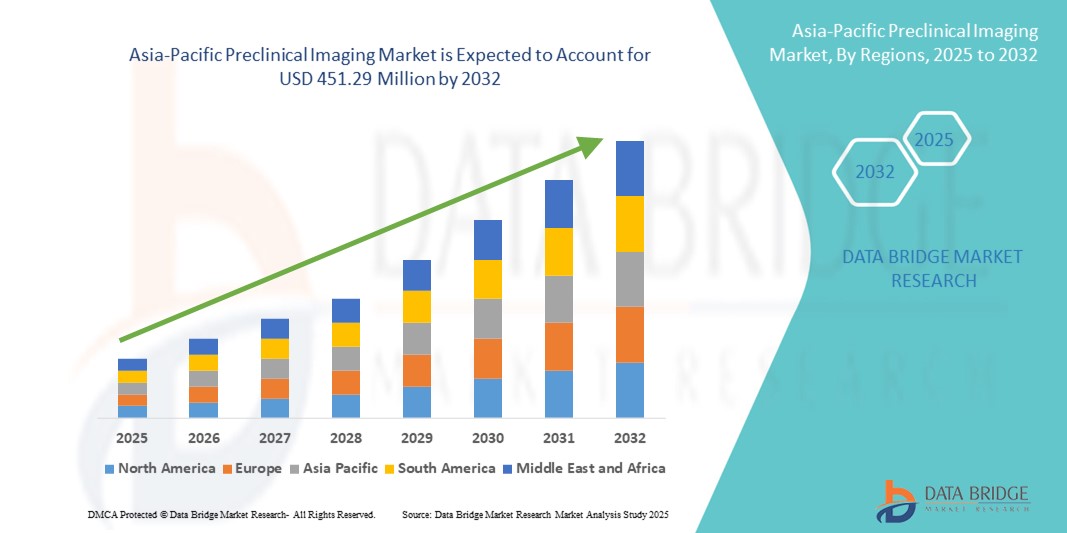
Asia-Pacific Preclinical Imaging Market Size
- The Asia-Pacific preclinical imaging market size was valued at USD 294.06 million in 2024 and is expected to reach USD 451.29 million by 2032, at a CAGR of 5.50% during the forecast period
- The market growth is largely driven by increasing adoption of advanced imaging modalities and growing investment in preclinical research and drug discovery across the region, particularly in countries such as China, Japan, and India
- Furthermore, rising focus on translational research, early disease diagnosis, and non-invasive imaging techniques is encouraging the use of preclinical imaging systems in pharmaceutical and biotechnology research. These converging factors are accelerating the uptake of innovative imaging solutions, thereby significantly boosting the industry's growth
Asia-Pacific Preclinical Imaging Market Analysis
- Preclinical imaging, encompassing advanced modalities such as MRI, CT, PET, SPECT, and optical imaging, is becoming an essential component of drug discovery, translational research, and early disease diagnosis across academic, pharmaceutical, and biotechnology research settings due to its non-invasive capabilities, high-resolution imaging, and ability to accelerate preclinical studies
- The escalating demand for preclinical imaging is primarily fueled by increasing investments in pharmaceutical R&D, growing focus on precision medicine, and rising adoption of advanced imaging technologies to reduce drug development timelines and enhance translational research outcomes
- China dominated the Asia-Pacific preclinical imaging market with the largest revenue share of 39% in 2024, characterized by substantial government funding for biomedical research, rapid expansion of pharmaceutical manufacturing, and adoption of advanced imaging systems in oncology, neurology, and biomarker studies
- India is expected to be the fastest-growing country in the Asia-Pacific preclinical imaging market during the forecast period, driven by increasing clinical trial volumes, rising pharmaceutical and biotechnology research activities, and growing adoption of cost-effective imaging solutions
- Preclinical optical imaging reagents segment dominated the Asia-Pacific preclinical imaging market with a market share of 38.7% in 2024, due to their widespread application in molecular imaging, cancer research, and in vivo disease modeling
Report Scope and Asia-Pacific Preclinical Imaging Market Segmentation
|
Attributes |
Asia-Pacific Preclinical Imaging Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Preclinical Imaging Market Trends
Advancements in Multimodal Imaging and AI Integration
- A significant and accelerating trend in the Asia-Pacific preclinical imaging market is the integration of multimodal imaging systems with artificial intelligence (AI) and advanced data analytics platforms. This combination is enhancing image resolution, accelerating analysis, and enabling more accurate and predictive preclinical studies
- For instance, the combination of PET/MRI systems with AI-driven image reconstruction in China allows researchers to conduct longitudinal studies with higher precision and reduced scan times. Similarly, Japan has witnessed adoption of AI-powered optical imaging platforms for high-throughput cancer research, improving early detection and therapeutic evaluation
- AI integration enables automated image segmentation, pattern recognition, and predictive modeling, which assists in better understanding disease progression and drug efficacy. For instance, AI-assisted MRI analysis in India improves biomarker identification and reduces manual interpretation errors, while multimodal imaging provides comprehensive insights by combining anatomical and functional data
- The seamless integration of imaging systems with centralized laboratory information management platforms allows researchers to manage data from multiple preclinical studies, facilitating high-throughput workflows and reproducible results
- This trend toward more intelligent, precise, and interconnected imaging solutions is fundamentally reshaping preclinical research standards. Consequently, companies such as MILabs and Bruker are developing AI-enabled preclinical imaging systems with multimodal functionality and enhanced imaging analytics capabilities
- The demand for preclinical imaging systems with AI and multimodal integration is growing rapidly across pharmaceutical, biotech, and academic research sectors, as these technologies accelerate drug discovery and improve translational research outcomes
Asia-Pacific Preclinical Imaging Market Dynamics
Driver
Rising Investment in Pharmaceutical R&D and Translational Research
- The increasing investment in pharmaceutical and biotechnology R&D across Asia-Pacific, particularly in China, Japan, and India, is a major driver of preclinical imaging adoption. Advanced imaging systems support early-stage drug development, biomarker validation, and disease modeling
- For instance, in March 2024, a leading Chinese research consortium expanded its preclinical imaging infrastructure to support oncology drug trials, integrating PET/CT and MRI systems with AI-based analysis tools
- As pharmaceutical companies seek to reduce drug development timelines and costs, preclinical imaging offers non-invasive, high-resolution insights into disease progression and therapeutic efficacy
- The growing focus on precision medicine, early disease detection, and translational research is increasing demand for integrated, high-performance imaging systems in both academic and commercial research settings
- Government initiatives and grants in countries such as Japan and South Korea aimed at supporting biomedical research are providing additional funding for imaging infrastructure, further accelerating market growth
- Collaborations between imaging equipment manufacturers and research institutes for co-development of specialized imaging solutions are opening new market opportunities
Restraint/Challenge
High Equipment Cost and Skilled Workforce Requirement
- The high cost of advanced preclinical imaging systems and reagents remains a significant barrier to widespread adoption, especially for smaller research institutes and emerging biotech firms. Systems such as PET/MRI or multimodal optical imaging require substantial capital investment
- Furthermore, operating complex imaging systems demands highly trained personnel for image acquisition, analysis, and maintenance. A shortage of skilled imaging specialists in emerging markets such as India and Southeast Asia can slow market penetration
- For instance, a 2024 report highlighted that several mid-sized biotech labs in India delayed PET/CT adoption due to both budget constraints and lack of trained operators
- While some companies are offering smaller, cost-effective imaging solutions, premium multimodal systems with AI integration continue to carry high price tags, limiting accessibility
- Regulatory compliance and approvals for preclinical imaging equipment, particularly in China and Japan, can slow deployment and increase operational costs
- Data management and integration challenges, including handling large volumes of imaging data securely and efficiently, remain a barrier for smaller institutions lacking IT infrastructure
- Overcoming these challenges through training programs, collaborative research initiatives, and development of affordable imaging platforms will be crucial for sustained market growth in the Asia-Pacific preclinical imaging sector
Asia-Pacific Preclinical Imaging Market Scope
The market is segmented on the basis of product, reagents, application, and end user.
- By Product
On the basis of product, the Asia-Pacific preclinical imaging market is segmented into systems and services. The systems segment dominated the market with the largest revenue share of 65.4% in 2024, driven by the growing adoption of advanced imaging modalities such as MRI, CT, PET, SPECT, and optical imaging in preclinical research. High-resolution imaging systems are increasingly preferred by pharmaceutical companies and academic institutions for detailed anatomical and functional studies. The reliability, reproducibility, and long-term utility of systems make them indispensable for drug discovery and translational research. Research institutes prioritize systems for their ability to support multimodal imaging studies and longitudinal experiments. In addition, the rising focus on precision medicine and biomarker identification is further accelerating the adoption of imaging systems in the region.
The services segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing outsourcing of imaging studies to specialized preclinical service providers. Services allow smaller laboratories and CROs to access advanced imaging technologies without the burden of heavy capital investment. Preclinical imaging service providers offer comprehensive solutions, including imaging, data analysis, and report generation, which support rapid decision-making in drug development. The segment benefits from the increasing demand for contract-based preclinical studies in oncology, neurology, and cardiovascular research. Furthermore, flexible service models and customized imaging solutions are encouraging adoption across emerging markets in Asia-Pacific. The cost-effectiveness and convenience of these services make them attractive to a broader base of end users.
- By Reagents
On the basis of reagents, the Asia-Pacific preclinical imaging market is segmented into preclinical optical imaging reagents, preclinical nuclear imaging reagents, preclinical MRI contrast agents, preclinical ultrasound contrast agents, and preclinical CT contrast agents. Preclinical optical imaging reagents dominated the market with a share of 38.7% in 2024, owing to their extensive application in molecular imaging, cancer research, and in vivo disease modeling. These reagents are widely used for longitudinal studies, allowing repeated imaging of the same subject to monitor disease progression or therapeutic response. Their compatibility with multimodal imaging systems enhances their utility in translational research. Optical imaging reagents also enable high-sensitivity detection of specific biomolecules and cellular events in preclinical models. The ability to visualize molecular interactions in live animals supports rapid drug development and biomarker validation.
Preclinical MRI contrast agents are expected to witness the fastest growth during the forecast period, driven by the rising adoption of MRI for soft tissue imaging and biomarker evaluation. Innovations in contrast agents that enhance resolution, reduce toxicity, and provide tissue-specific imaging are boosting market expansion. Increasing use of MRI contrast agents in longitudinal studies allows researchers to track changes in tissue structure and function over time. Pharmaceutical companies are increasingly using advanced MRI agents to study disease progression and therapeutic efficacy. Research collaborations with imaging equipment manufacturers are accelerating the development of next-generation MRI contrast reagents. Furthermore, government support for biomedical research in Asia-Pacific is contributing to the adoption of these agents in preclinical studies.
- By Application
On the basis of application, the Asia-Pacific preclinical imaging market is segmented into research and development, drug discovery, bio-distribution, cancer cell detection, bio-markers, and others. The research and development segment dominated the market with a share of 44.5% in 2024, supported by increasing investment in early-stage drug discovery and translational research. High adoption of imaging systems in academic and pharmaceutical R&D allows researchers to study disease models in vivo and evaluate drug efficacy in real-time. Preclinical imaging facilitates detailed mechanistic studies, helping to optimize drug candidates before clinical trials. The integration of AI and advanced image analysis software is improving the efficiency and accuracy of R&D workflows. Researchers increasingly rely on imaging to validate therapeutic targets, analyze pharmacokinetics, and assess toxicity. This has positioned preclinical imaging as a cornerstone of modern biomedical research in Asia-Pacific.
The cancer cell detection segment is expected to witness the fastest CAGR from 2025 to 2032, due to rising prevalence of cancer and the critical role of preclinical imaging in evaluating anti-cancer therapies. Imaging modalities enable tracking of tumor growth, metastasis, and response to treatment in animal models. The use of molecular and optical imaging agents allows precise visualization of cancer cells at early stages. Pharmaceutical companies rely on preclinical imaging to test novel therapeutic compounds and immunotherapies. Increasing focus on personalized medicine and targeted therapy is further fueling demand. Additionally, partnerships between imaging reagent developers and research institutes are enhancing capabilities for high-sensitivity cancer cell detection.
- By End User
On the basis of end user, the Asia-Pacific preclinical imaging market is segmented into contract research organizations (CROs), pharmaceutical & biotech companies, academic & government research institutes, diagnostics centers, and others. Pharmaceutical & biotech companies dominated the market with a share of 47.1% in 2024, owing to their significant R&D expenditure and growing demand for early-stage drug evaluation. These companies utilize preclinical imaging for drug candidate screening, toxicity assessment, and biomarker validation. Imaging systems enable faster go/no-go decisions, reducing development costs and timelines. Integration with automated and high-throughput platforms further strengthens their adoption. The growing trend of precision medicine and targeted therapies has increased reliance on imaging technologies for preclinical studies. Collaborations with imaging equipment and reagent manufacturers also support expansion in this segment.
Academic & government research institutes are expected to witness the fastest growth during the forecast period, driven by increasing government funding for biomedical research and expansion of research infrastructure. Institutes are adopting preclinical imaging to advance fundamental research, study disease mechanisms, and develop new therapeutic strategies. Partnerships with global imaging equipment providers allow access to high-resolution systems and cutting-edge reagents. Training programs and collaborative research projects are promoting wider use of imaging across academic labs. The adoption of multimodal imaging and AI-based analysis tools is further enhancing research capabilities.
Asia-Pacific Preclinical Imaging Market Regional Analysis
- China dominated the Asia-Pacific preclinical imaging market with the largest revenue share of 39% in 2024, characterized by substantial government funding for biomedical research, rapid expansion of pharmaceutical manufacturing, and adoption of advanced imaging systems in oncology, neurology, and biomarker studies
- Researchers and institutions in the region highly value the high-resolution, non-invasive imaging capabilities provided by preclinical systems, which accelerate drug discovery, translational research, and early disease detection
- This widespread adoption is further supported by rising investment in pharmaceutical and biotechnology R&D, increasing clinical trial volumes, and the growing focus on precision medicine, establishing preclinical imaging as a critical tool for both academic and commercial research organizations across Asia-Pacific
The China Preclinical Imaging Market Insight
The China preclinical imaging market captured the largest revenue share in Asia-Pacific in 2024, fueled by extensive government funding for biomedical research, rapid expansion of pharmaceutical manufacturing, and increasing adoption of multimodal imaging systems in oncology, neurology, and biomarker studies. The country’s emphasis on high-tech research and translational drug development is promoting the deployment of advanced imaging solutions across academic and commercial laboratories.
Japan Preclinical Imaging Market Insight
The Japan preclinical imaging market is gaining momentum due to its strong R&D ecosystem, high technological adoption, and focus on precision medicine. Adoption is further driven by the integration of AI and multimodal imaging in preclinical studies, enabling more accurate disease modeling and therapeutic evaluation. The country’s well-established research infrastructure and government support for biomedical innovation continue to boost market growth.
India Preclinical Imaging Market Insight
The India preclinical imaging market is expected to witness the fastest growth in Asia-Pacific during the forecast period, attributed to rapid expansion of research infrastructure, increasing clinical trial activity, and rising adoption of cost-effective imaging systems. Government initiatives promoting biomedical research, the emergence of CROs, and collaborations with global imaging technology providers are fueling demand in both academic and pharmaceutical sectors.
South Korea Preclinical Imaging Market Insight
The South Korea preclinical imaging market is expanding steadily, supported by growing investment in biotechnology R&D, advanced imaging adoption in cancer and neurological research, and increasing collaborations between local research institutes and global imaging system manufacturers. The country’s focus on digital health and innovative imaging solutions is encouraging wider market penetration.
Asia-Pacific Preclinical Imaging Market Share
The Asia-Pacific preclinical imaging industry is primarily led by well-established companies, including:
- Bruker (U.S.)
- PerkinElmer (U.S.)
- FUJIFILM VisualSonics, Inc. (Canada)
- Aspect Imaging Ltd. (Israel)
- TriFoil Imaging (U.S.)
- LI-COR Biosciences (U.S.)
- Mediso Ltd. (Hungary)
- MILabs B.V. (Netherlands)
- MR Solutions Ltd. (U.K.)
- GE Healthcare (U.S.)
- Siemens Healthineers AG (Germany)
- Canon Medical Systems Corporation (Japan)
- Shanghai United Imaging Healthcare Co., Ltd. (China)
- Mindray Medical International Limited (China)
- Neusoft Medical Systems Co., Ltd. (China)
- Hitachi Medical Corporation (Japan)
- Koninklijke Philips N.V. (Netherlands)
- Olympus Corporation (Japan)
- Hamamatsu Photonics K.K. (Japan)
- 3DHISTECH Ltd. (Hungary)
What are the Recent Developments in Asia-Pacific Preclinical Imaging Market?
- In December 2024, Intas Pharmaceuticals announced an agreement to acquire the UDENYCA (pegfilgrastim-cbqv) business from Coherus BioSciences, Inc. for up to USD 558 million. The acquisition, which was completed in the first quarter of 2025, includes the biosimilar drug and all related assets. UDENYCA is a biosimilar to Neulasta, used to treat chemotherapy-induced neutropenia. This strategic move strengthens Intas's biosimilar portfolio and solidifies its position as a major global supplier of pegfilgrastim
- In October 2024, the World Health Organization (WHO) hosted a Pharmacovigilance Partners' Meeting in New Delhi, India. The meeting, held during the 19th International Conference of Drug Regulatory Authorities (ICDRA), brought together regulators from 68 countries to review a draft of the WHO Global Smart Pharmacovigilance Strategy. The goal of this initiative is to promote a convergent evolution of pharmacovigilance activities among member countries, leading to harmonized and pragmatic regulatory requirements
- In April 2024, WuXi STA, a subsidiary of WuXi AppTec, detailed a multi-site, global expansion plan, including a new 169-acre active pharmaceutical ingredient (API) manufacturing facility in Taixing, China. This expansion, along with other facilities, aims to enhance manufacturing capabilities and capacities to meet the growing demand for drug development services, including preclinical studies, in the Asia-Pacific region and globally
- In November 2022, Bruker Corporation announced the acquisition of Inscopix, Inc., a neuroscience company specializing in miniaturized microscopes, or "miniscopes," for brain imaging in freely moving animals. This acquisition enhances Bruker's neuroscience portfolio by adding products and services that allow for a deeper exploration of neural network function in animals, which is crucial for understanding neurological disorders
- In May 2022, FUJIFILM VisualSonics Inc. launched the Vevo F2, which is the first ultra-high to low-frequency (71MHz-1MHz) ultrasound and photoacoustic imaging system in the world for preclinical use. This system features HD image processing technology and a completely new signal pathway from transducer to display, enabling better image clarity and higher frame rates. This advancement is particularly suited for cross-functional biological and physiological research, including oncology, developmental biology, neurobiology, and cardiology
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.