Asia Pacific Medical Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
5.52 Billion
USD
9.01 Billion
2024
2032
USD
5.52 Billion
USD
9.01 Billion
2024
2032
| 2025 –2032 | |
| USD 5.52 Billion | |
| USD 9.01 Billion | |
|
|
|
|
Asia-Pacific Medical Devices Market Size
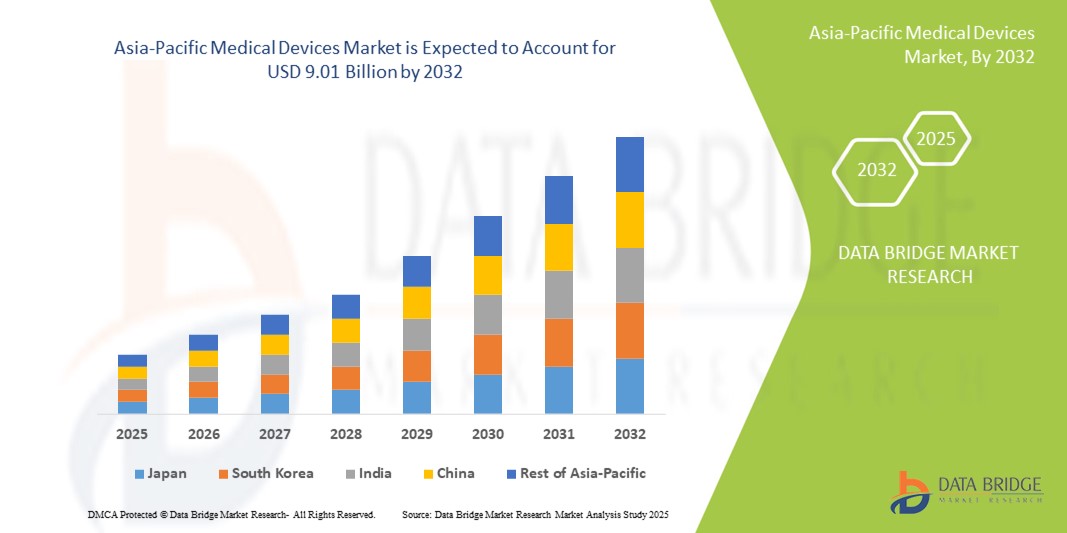
- The Asia-Pacific medical devices market size was valued at USD 5.52 billion in 2024 and is expected to reach USD 9.01 billion by 2032, at a CAGR of 6.30% during the forecast period
- The market growth is largely fueled by the rapid urbanization, expanding healthcare infrastructure, and technological advancements in medical diagnostics and therapeutic equipment across emerging economies in the Asia-Pacific region, leading to enhanced accessibility and modernization of healthcare delivery systems
- Furthermore, rising demand for affordable, portable, and minimally invasive medical devices, coupled with increasing government investments and favorable regulatory reforms, is establishing the Asia-Pacific region as a key growth hub in the global medical devices market. These converging factors are accelerating the adoption of innovative medical technologies, thereby significantly boosting the industry’s growth across countries such as China, India, Japan, and South Korea
Asia-Pacific Medical Devices Market Analysis
- Medical devices, including diagnostic, therapeutic, and monitoring equipment, are becoming increasingly essential in Asia-Pacific due to rising chronic disease prevalence, expanding healthcare access, and a growing focus on early and accurate diagnosis. Rapid technological advancements and increased healthcare expenditure across both public and private sectors are further accelerating the adoption of innovative medical devices in hospitals, clinics, and homecare settings
- The surging demand for medical devices in Asia-Pacific is primarily driven by the aging population, an increase in respiratory and cardiovascular conditions, and the widespread need for efficient home-based care solutions. The rising prevalence of conditions such as COPD, asthma, and sleep apnea is fueling demand for devices such as ventilators, CPAP/BIPAP, and oxygen concentrators
- China dominated the Asia-Pacific medical devices market with the largest market share of 39.6% in 2024, driven by a large patient base, fast-growing healthcare digitization, and strong domestic production of affordable yet advanced medical technologies. Government initiatives supporting rural healthcare modernization and chronic disease screening programs are also key contributors
- India is expected to be the fastest growing region in the Asia-Pacific medical devices market between 2025 and 2032. Factors such as increasing healthcare investments, expansion of private hospitals, government initiatives such as Make in India, and rising awareness of joint and trauma-related treatments are significantly propelling market growth
- The reconstructive joint replacements segment dominated the Asia-Pacific medical devices market with the largest revenue share of 32.8% in 2024, owing to the rising incidence of osteoarthritis and rheumatoid arthritis, along with a growing geriatric population requiring knee and hip replacements
Report Scope and Asia-Pacific Medical Devices Market Segmentation
|
Attributes |
Asia-Pacific Medical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Medical Devices Market Trends
“Increasing Demand for Intelligent and Interconnected Healthcare Solutions”
- A significant and accelerating trend in the Asia-Pacific medical devices market is the deepening integration of advanced technologies such as artificial intelligence (AI) and Internet of Things (IoT) to enhance functionality, accuracy, and user experience across various devices. These innovations are streamlining healthcare delivery and enabling real-time data-driven decision-making in clinical and home settings
- For instance, AI-enabled ventilators and portable oxygen concentrators are being adopted to automatically adjust respiratory support based on patient vitals. Similarly, smart CPAP/BiPAP devices are providing real-time feedback and compliance tracking, empowering both patients and providers to optimize therapy outcomes
- The integration of medical devices with mobile applications and cloud-based platforms allows remote patient monitoring, early diagnostics, and preventive care, which is particularly beneficial in rural or underserved regions. This digital transformation is creating a more connected healthcare ecosystem across Asia-Pacific
- Technologies that enable centralized control and interoperability between medical devices and electronic health records (EHRs) are also improving workflow efficiency and reducing administrative burdens in hospitals and clinics
- The growing availability of user-friendly, intelligent healthcare equipment is reshaping expectations for both patients and providers. Consequently, companies in the region are developing smarter, more accessible medical devices with features such as automated alerts, remote adjustments, and health tracking dashboards
- The demand for integrated, data-driven medical solutions is growing rapidly across the Asia-Pacific region, driven by rising healthcare awareness, increasing chronic disease burden, and government support for digital health initiatives
Asia-Pacific Medical Devices Market Dynamics
Driver
“Growing Need Due to Rising Healthcare Demands and Technological Adoption”
- The increasing burden of chronic diseases, aging population, and the demand for improved healthcare infrastructure are key drivers accelerating the adoption of advanced medical devices across the Asia-Pacific region. Rapid urbanization and healthcare awareness are further pushing governments and private sectors to invest in modern diagnostic and therapeutic equipment
- For instance, in March 2024, Japan’s Ministry of Health approved funding for next-generation ventilators and portable diagnostic devices to enhance home-based care and pandemic preparedness. Such initiatives by public bodies and private players are expected to drive growth in the Asia-Pacific Medical Devices Market over the forecast period
- Patients and providers are becoming more aware of the benefits of early diagnosis and preventive care, driving the adoption of technologies such as portable oxygen concentrators, digital spirometers, and AI-integrated CPAP/BiPAP machines that offer superior patient outcomes
- Furthermore, the growing popularity of telehealth services and the trend toward decentralized care are making medical devices more crucial in homecare settings. Devices that integrate easily with mobile health platforms and electronic health records (EHRs) are becoming essential tools for remote patient monitoring
- The shift toward portable, user-friendly, and efficient healthcare equipment is encouraging adoption across both large hospitals and small clinics. With expanding health insurance coverage and government support for digital health, medical devices are becoming more accessible to the general population in countries such as India, China, and Southeast Asian nations
Restraint/Challenge
“Concerns Regarding Regulatory Complexity and High Initial Costs”
- The Asia-Pacific medical devices Market faces challenges related to varying regulatory frameworks across countries, which can hinder product approvals and market entry. Manufacturers must navigate different compliance requirements in markets such as China, India, and Japan, adding complexity and cost to their operations
- For instance, the implementation of China’s new Medical Device Regulations (MDR) in 2021 increased requirements for clinical evidence, which can delay product launches and add to development costs
- In addition, the high upfront cost of sophisticated medical equipment such as ventilators, anesthesia machines, and diagnostic imaging systems can be a barrier for smaller healthcare providers and facilities in developing regions. Budget limitations and lack of access to financing options limit widespread adoption in rural and underserved areas
- While prices are gradually decreasing and local manufacturing is rising, the perceived high cost of premium medical devices remains a concern, particularly for long-term care and small-scale facilities
- Overcoming these challenges will require harmonized regulatory processes, increased investment in local manufacturing, government subsidies, and awareness campaigns to improve affordability and trust in new medical technologies
Asia-Pacific Medical Devices Market Scope
The market is segmented on the basis of product, device type, biomaterial, procedures, and end user.
• By Product
On the basis of product, the Asia-Pacific medical devices market is segmented into reconstructive joint replacements, spinal implants, trauma and craniomaxillofacial, dental implants, and orthobiologics. The reconstructive joint replacements segment dominated the market with the largest revenue share of 32.8% in 2024, owing to the rising incidence of osteoarthritis and rheumatoid arthritis, along with a growing geriatric population requiring knee and hip replacements.
The dental implants segment is projected to witness the fastest CAGR of 24.1% from 2025 to 2032, driven by increasing dental tourism, rising awareness of oral health, and advancements in implant materials and techniques.
• By Device Type
On the basis of device type, the Asia-Pacific medical devices market is segmented into internal fixation devices and external fixation devices. The internal fixation devices segment accounted for the largest market share of 58.5% in 2024, due to the high preference for internal stabilization in fracture treatments and spinal fixation, which promotes better healing and reduced hospitalization time.
The external fixation devices segment is expected to grow at a steady CAGR, supported by increased application in trauma care and orthopedic emergency interventions.
• By Biomaterial
On the basis of biomaterial, the Asia-Pacific medical devices market is segmented into metallic biomaterials, polymeric biomaterials, ceramic biomaterials, natural biomaterials, and others. Metallic biomaterials held the largest share at 41.3% in 2024, attributed to their widespread use in load-bearing implants such as joint replacements and spinal fixation due to superior strength and durability.
Polymeric biomaterials are expected to record the fastest CAGR of 22.6%, owing to their rising adoption in tissue engineering, drug delivery, and bioresorbable devices.
• By Procedures
On the basis of procedures, the Asia-Pacific medical devices market is segmented into open surgery and minimally invasive surgery (MIS). Open surgery captured the largest share of 56.4% in 2024, largely due to its continued use in complex orthopedic reconstructions and trauma care.
Minimally invasive surgery (MIS) is anticipated to grow at the fastest CAGR of 27.8% from 2025 to 2032, fueled by benefits such as reduced recovery time, less postoperative pain, and improved surgical precision.
• By End User
On the basis of end users, the Asia-Pacific medical devices market is segmented into hospitals, ambulatory care centers, specialized clinics, orthopedic centers, and others. Hospitals led the market with the highest revenue share of 48.9% in 2024, due to their comprehensive service offerings, advanced infrastructure, and higher patient footfall for surgical interventions.
Orthopedic centers are expected to grow at the fastest CAGR, driven by the increasing establishment of dedicated orthopedic and rehabilitation facilities across developing countries in the region.
Asia-Pacific Medical Devices Market Regional Analysis
- Asia-Pacific dominated the global medical devices market with the largest revenue share of 34.80% in 2024, driven by increasing healthcare expenditures, rapid urbanization, and an expanding geriatric population that requires orthopedic and diagnostic interventions
- Regional governments are heavily investing in improving healthcare infrastructure, while rising medical tourism, particularly in countries such as India, Thailand, and Malaysia, further fuels demand for advanced medical technologies
- Growing awareness of minimally invasive procedures, increased accessibility to medical facilities, and favorable reimbursement policies are also major contributors to the region’s market growth
China Asia-Pacific Medical Devices Market Insight
The China medical devices market held the largest share with 39.6% in Asia-Pacific in 2024, attributed to the country's massive population base, rising elderly demographic, and increased demand for orthopedic procedures. Strategic efforts by domestic manufacturers and favorable government healthcare reforms have accelerated the availability and affordability of cutting-edge medical devices.
Japan Asia-Pacific Medical Devices Market Insight
The Japan medical devices market continues to grow due to its strong technological foundation, an aging population, and a robust preference for high-quality and precise healthcare solutions. Japan’s commitment to innovation and early adoption of advanced surgical techniques supports increased usage of minimally invasive and reconstructive devices.
India Asia-Pacific Medical Devices Market Insight
The India medical devices market is expected to witness the fastest CAGR between 2025 and 2032. Factors such as increasing healthcare investments, expansion of private hospitals, government initiatives such as Make in India, and rising awareness of joint and trauma-related treatments are significantly propelling market growth.
Asia-Pacific Medical Devices Market Share
The Asia-Pacific medical devices industry is primarily led by well-established companies, including:
- Zimmer Biomet (U.S.)
- Smith + Nephew (U.K.)
- Medtronic (Ireland)
- Stryker (U.S.)
- B. Braun SE (Germany)
- NuVasive, Inc. (U.S.)
- ENOVIS CORPORATION (U.S.)
- Institut Straumann AG (Switzerland)
- OSSTEM IMPLANT CO., LTD. (South Korea)
- Narang Medical Limited (U.S.)
- Globus Medical (U.S.)
- Arthrex, Inc. (U.S.)
- CONMED Corporation (U.S.)
- Integra LifeSciences Corporation (U.S.)
- RTI Surgical (U.S.)
- W. L. Gore & Associates, Inc. (U.S.)
- Corin Group (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
Latest Developments in Asia-Pacific Medical Devices Market
- In May 2024, Smith & Nephew plc opened a new R&D and manufacturing center in Malaysia to support the growing demand for orthopedic and wound care products in the Asia-Pacific region. This facility enhances the company’s regional capabilities, underlining its commitment to expanding access to advanced medical devices across Southeast Asia and boosting local production efficiency
- In April 2024, Stryker launched its Mako SmartRobotics system in several hospitals across Japan and Australia, enabling precise and minimally invasive joint replacement surgeries. This move reinforces Stryker’s presence in the region and showcases the growing adoption of robotic-assisted surgical technologies in APAC healthcare systems
- In March 2024, Medtronic partnered with India’s Apollo Hospitals to expand access to its Micra AV pacemaker, a miniaturized device designed for atrioventricular (AV) block treatment. This collaboration supports the growing need for innovative cardiac care in India and reflects Medtronic’s strategy to improve patient outcomes in emerging markets
- In February 2024, Zimmer Biomet announced the launch of its Persona IQ smart knee system in select hospitals across South Korea. This device combines orthopedic implants with smart sensor technology, providing surgeons and patients with real-time data insights for postoperative monitoring and rehabilitation
- In January 2024, Fisher & Paykel Healthcare expanded its respiratory care product line in China by introducing the Airvo 3 high-flow system, addressing the region’s increasing demand for non-invasive oxygen therapy solutions. The initiative demonstrates the company’s focus on innovation and tailored healthcare solutions for the APAC market
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA-PACIFIC MEDICAL DEVICE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL SWINE AND POULTRY RESPIRATORY DISEASES TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 ASIA-PACIFIC MEDICAL DEVICE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR ASIA-PACIFIC MEDICAL DEVICE MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY PRODUCT TYPE
16.1 OVERVIEW
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS OF PRODUCTS)
16.2 RESPIRATORY DEVICES
16.2.1 THERAPEUTIC
16.2.1.1. VENTILATOR
16.2.1.1.1. MARKET VALUE (USD)
16.2.1.1.2. MARKET VOLUME (UNIT)
16.2.1.1.3. ASP (USD)
16.2.1.2. MASK
16.2.1.3. PAP DEVICE
16.2.1.4. INHALER
16.2.1.5. NEB
16.2.1.6. ULIZER
16.2.2 MONITORING
16.2.2.1. PULSE OXIMETER
16.2.2.1.1. MARKET VALUE (USD)
16.2.2.1.2. MARKET VOLUME (UNIT)
16.2.2.1.3. ASP (USD)
16.2.3 CAPNOGRAPH
16.2.3.1. MARKET VALUE (USD)
16.2.3.2. MARKET VOLUME (UNIT)
16.2.3.3. ASP (USD)
16.2.4 DIAGNOSTIC
16.2.4.1. MARKET VALUE (USD)
16.2.4.2. MARKET VOLUME (UNIT)
16.2.4.3. ASP (USD)
16.2.5 CONSUMABLES
16.2.5.1. MARKET VALUE (USD)
16.2.5.2. MARKET VOLUME (UNIT)
16.2.5.3. ASP (USD)
16.3 DIAGNOSTIC DEVICES
16.3.1 ELECTRODIAGNOSTIC DEVICE
16.3.1.1. ULTRASOUND SYSTEMS
16.3.1.2. MAGNETIC RESONANCE IMAGING (MRI)
16.3.1.3. ELECTROCARDIOGRAPHS
16.3.1.4. SCINTIGRAPHIC APPARATUS
16.3.1.5. OTHER ELECTRODIAGNOSTIC DEVICE
16.3.2 RADIATION DEVICE
16.3.2.1. SMARTWATCHES WITH HEALTH MONITORING
16.3.2.2. WEARABLE BLOOD PRESSURE MONITORS
16.3.2.3. REMOTE MONITORING SYSTEMS
16.3.2.4. TELEMEDICINE DEVICES
16.3.2.5. CT SCANNER16.3.2.6.
16.3.3 IMAGING PARTS & ACCESSORIES
16.3.3.1. CONTRAST MEDIA
16.3.3.2. X-RAY TUBES
16.3.3.3. MEDICAL X-RAY FILM16.3.3.4.
16.4 CARDIOVASCULAR DEVICES
16.4.1 ELECTROCARDIOGRAM (ECG)
16.4.1.1. REMOTE CARDIAC MONITORING
16.4.1.2. OTHER DIAGNOSTIC AND MONITORING DEVICE
16.4.2 THERAPEUTIC AND SURGICAL DEVICE
16.4.2.1. CARDIAC ASSIST DEVICE
16.4.2.2. CARDIAC RHYTHM MANAGEMENT DEVICE
16.4.2.3. CATHETER
16.4.2.4. GRAFTS
16.4.2.5. HEART VALVES
16.4.2.6. STENTS
16.4.2.7. OTHER THERAPEUTIC AND SURGICAL DEVICE
16.5 DENTAL
16.5.1 DENTAL INSTRUMENT & SUPPLIES
16.5.1.1. DENTAL INSTRUMENTS
16.5.1.2. DENTAL CEMENTS
16.5.1.3. TEETH & OTHER FITTINGS
16.5.2 DENTAL CAPITAL EQUIPMENT
16.5.2.1. DENTAL DRILLS
16.5.2.2. DENTAL X-RAY
16.5.2.3. DENTAL CHAIRS
16.6 ORTHOPEDIC DEVICES
16.6.1 FIXATION DEVICE
16.6.2 ARTIFICIAL JOINTS
16.6.3 OTHER ARTIFICIAL BODY PARTS
16.7 ENDOSCOPY DEVICES
16.7.1 VISUALIZATION EQUIPMENT
16.7.1.1. ENDOSCOPIC CAMERA
16.7.1.2. SD VISUALIZATION SYSTEM
16.7.1.3. HD VISUALIZATION SYSTEM
16.7.2 ENDOSCOPES
16.7.2.1. RIGID ENDOSCOPE
16.7.2.2. FLEXIBLE ENDOSCOPE
16.7.2.3. CAPSULE ENDOSCOPE
16.7.2.4. ROBOT-ASSISTED ENDOSCOPE
16.7.3 ENDOSCOPIC OPERATIVE DEVICE
16.7.3.1. IRRIGATION/SUCTION SYSTEM
16.7.3.2. ACCESS DEVICE
16.7.3.3. WOUND PROTECTOR
16.7.3.4. INSUFFLATION DEVICE
16.7.3.5. OPERATIVE MANUAL INSTRUMENT
16.7.3.6. OTHER ENDOSCOPIC OPERATIVE DEVICE
16.8 OPHTHALMOLOGY DEVICES
16.9 RADIOTHERAPY DEVICES
16.1 AESTHETIC DEVICES
16.10.1 LABORATORY EQUIPMENT
16.10.1.1. GENERAL EQUIPMENT
16.10.1.2. INCUBATORS
16.10.1.3. CENTRIFUGES
16.10.1.4. LABORATORY HOOD
16.10.1.5. AUTOCLAVE
16.10.1.6. SCOPES
16.10.1.7. SONICATORS
16.10.1.8. OTHERS
16.11 ANALYTICAL EQUIPMENT
16.11.1 SPECTROMETER
16.11.1.1. MASS SPECTROMETER
16.11.1.2. FLUORESCENCE SPECTROMETER
16.11.1.3. INFRARED SPECTROMETER
16.11.1.4. OTHERS
16.11.2 ANALYZER
16.11.2.1. ELEMENTAL ANALYZERS
16.11.2.2. PARTICLE SIZE ANALYZERS
16.11.2.3. OTHERS
16.11.3 TITRATORS
16.11.4 RHEOMETERS
16.11.5 FLOW INJECTION SYSTEM
16.11.6 SAMPLE PREPARATION SYSTEM
16.11.7 CHROMATOGRAPHY EQUIPMENT
16.11.7.1. GAS CHROMATOGRAPHY EQUIPMENT
16.11.7.2. LIQUID CHROMATOGRAPHY EQUIPMENT
16.11.8 OTHERS
16.12 SUPPORT EQUIPMENT
16.12.1 CELL HARVESTERS
16.12.2 RADIOMETRIC DETECTORS
16.12.3 MICROPLATE READERS
16.12.4 OTHERS
16.13 SPECIALTY EQUIPMENT
16.13.1 CYTOGENETICS INSTRUMENTS
16.13.2 CELL IMAGING DEVICE
16.13.3 LABORATORY EVAPORATORS
16.13.4 POLARIMETERS
16.13.5 MEMBRANE FILTRATION SYSTEMS
16.13.6 LASER SYSTEMS
16.13.7 OTHERS
16.14 SPECTROMETERS
16.15 OTHERS
17 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY DEVICE CLASS
17.1 CLASS I
17.2 CLASS II
17.3 CLAS III
17.4 CLASS IV
18 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY TYPE
18.1 INVASIVE DEVICE
18.2 NON-INVASIVE DEVICE
18.3 IMPLANTABLE DEVICE
18.4 WEARABLE DEVICE
18.5 OTHERS
19 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY APPLICATION
19.1 DIAGNOSTIC
19.2 THERAPEUTIC
20 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY MANUFACTURING METHOD
20.1 IN-HOUSE MANUFACTURING
20.2 OUTSOURCING
21 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS & CLINICS
21.2.1 IN-PATIENT
21.2.2 OUT-PATIENT
21.3 SPECIALTY CLINICS
21.4 AMBULATORY CARE CENTERS
21.5 BIOPHARMACEUTICAL COMPANIES
21.6 LABORATORIES
21.7 ACADEMICS & RESEARCH INSTITUTES
21.8 OTHERS
22 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 DISTRIBUTORS
22.4 RETAIL SALES
22.5 OTHERS
23 ASIA-PACIFIC MEDICAL DEVICE MARKET, BY GEOGRAPHY
ASIA-PACIFIC MEDICAL DEVICE MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 ASIA-PACIFIC
23.1.1 JAPAN
23.1.2 CHINA
23.1.3 SOUTH KOREA
23.1.4 INDIA
23.1.5 AUSTRALIA
23.1.6 SINGAPORE
23.1.7 THAILAND
23.1.8 MALAYSIA
23.1.9 INDONESIA
23.1.10 PHILIPPINES
23.1.11 REST OF ASIA-PACIFIC
23.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 ASIA-PACIFIC MEDICAL DEVICE MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 ASIA-PACIFIC MEDICAL DEVICE MARKET, SWOT AND DBMR ANALYSIS
26 ASIA-PACIFIC MEDICAL DEVICE MARKET, COMPANY PROFILE
26.1 MEDICAL DEVICE
26.1.1 MEDTRONIC PLC.
26.1.1.1. COMPANY OVERVIEW
26.1.1.2. REVENUE ANALYSIS
26.1.1.3. GEOGRAPHIC PRESENCE
26.1.1.4. PRODUCT PORTFOLIO
26.1.1.5. RECENT DEVELOPMENTS
26.1.2 BOSTON SCIENTIFIC CORPORATION
26.1.2.1. COMPANY OVERVIEW
26.1.2.2. REVENUE ANALYSIS
26.1.2.3. GEOGRAPHIC PRESENCE
26.1.2.4. PRODUCT PORTFOLIO
26.1.2.5. RECENT DEVELOPMENTS
26.1.3 JOHNSON & JOHNSON
26.1.3.1. COMPANY OVERVIEW
26.1.3.2. REVENUE ANALYSIS
26.1.3.3. GEOGRAPHIC PRESENCE
26.1.3.4. PRODUCT PORTFOLIO
26.1.3.5. RECENT DEVELOPMENTS
26.1.4 BECTON, DICKINSON AND COMPANY
26.1.4.1. COMPANY OVERVIEW
26.1.4.2. REVENUE ANALYSIS
26.1.4.3. GEOGRAPHIC PRESENCE
26.1.4.4. PRODUCT PORTFOLIO
26.1.4.5. RECENT DEVELOPMENTS
26.1.5 CARDINAL HEALTH
26.1.5.1. COMPANY OVERVIEW
26.1.5.2. REVENUE ANALYSIS
26.1.5.3. GEOGRAPHIC PRESENCE
26.1.5.4. PRODUCT PORTFOLIO
26.1.5.5. RECENT DEVELOPMENTS
26.1.6 STRYKER CORPORATION
26.1.6.1. COMPANY OVERVIEW
26.1.6.2. REVENUE ANALYSIS
26.1.6.3. GEOGRAPHIC PRESENCE
26.1.6.4. PRODUCT PORTFOLIO
26.1.6.5. RECENT DEVELOPMENTS
26.1.7 ABBOTT LABORATORIES
26.1.7.1. COMPANY OVERVIEW
26.1.7.2. REVENUE ANALYSIS
26.1.7.3. GEOGRAPHIC PRESENCE
26.1.7.4. PRODUCT PORTFOLIO
26.1.7.5. RECENT DEVELOPMENTS
26.1.8 BAXTER INTERNATIONAL
26.1.8.1. COMPANY OVERVIEW
26.1.8.2. REVENUE ANALYSIS
26.1.8.3. GEOGRAPHIC PRESENCE
26.1.8.4. PRODUCT PORTFOLIO
26.1.8.5. RECENT DEVELOPMENTS
26.1.9 DANAHER CORPORATION
26.1.9.1. COMPANY OVERVIEW
26.1.9.2. REVENUE ANALYSIS
26.1.9.3. GEOGRAPHIC PRESENCE
26.1.9.4. PRODUCT PORTFOLIO
26.1.9.5. RECENT DEVELOPMENTS
26.1.10 3M
26.1.10.1. COMPANY OVERVIEW
26.1.10.2. REVENUE ANALYSIS
26.1.10.3. GEOGRAPHIC PRESENCE
26.1.10.4. PRODUCT PORTFOLIO
26.1.10.5. RECENT DEVELOPMENTS
26.1.11 NOVARTIS AG
26.1.11.1. COMPANY OVERVIEW
26.1.11.2. REVENUE ANALYSIS
26.1.11.3. GEOGRAPHIC PRESENCE
26.1.11.4. PRODUCT PORTFOLIO
26.1.11.5. RECENT DEVELOPMENTS
26.1.12 GENERAL ELECTRIC COMPANY
26.1.12.1. COMPANY OVERVIEW
26.1.12.2. REVENUE ANALYSIS
26.1.12.3. GEOGRAPHIC PRESENCE
26.1.12.4. PRODUCT PORTFOLIO
26.1.12.5. RECENT DEVELOPMENTS
26.1.13 BD
26.1.13.1. COMPANY OVERVIEW
26.1.13.2. REVENUE ANALYSIS
26.1.13.3. GEOGRAPHIC PRESENCE
26.1.13.4. PRODUCT PORTFOLIO
26.1.13.5. RECENT DEVELOPMENTS
26.1.14 INTUITIVE SURGICAL
26.1.14.1. COMPANY OVERVIEW
26.1.14.2. REVENUE ANALYSIS
26.1.14.3. GEOGRAPHIC PRESENCE
26.1.14.4. PRODUCT PORTFOLIO
26.1.14.5. RECENT DEVELOPMENTS
26.1.15 ALLERGAN
26.1.15.1. COMPANY OVERVIEW
26.1.15.2. REVENUE ANALYSIS
26.1.15.3. GEOGRAPHIC PRESENCE
26.1.15.4. PRODUCT PORTFOLIO
26.1.15.5. RECENT DEVELOPMENTS
26.1.16 HOYA CORPORATION
26.1.16.1. COMPANY OVERVIEW
26.1.16.2. REVENUE ANALYSIS
26.1.16.3. GEOGRAPHIC PRESENCE
26.1.16.4. PRODUCT PORTFOLIO
26.1.16.5. RECENT DEVELOPMENTS
26.1.17 SIEMENS HEALTHCARE GMBH
26.1.17.1. COMPANY OVERVIEW
26.1.17.2. REVENUE ANALYSIS
26.1.17.3. GEOGRAPHIC PRESENCE
26.1.17.4. PRODUCT PORTFOLIO
26.1.17.5. RECENT DEVELOPMENTS
26.1.18 RESMED
26.1.18.1. COMPANY OVERVIEW
26.1.18.2. REVENUE ANALYSIS
26.1.18.3. GEOGRAPHIC PRESENCE
26.1.18.4. PRODUCT PORTFOLIO
26.1.18.5. RECENT DEVELOPMENTS
26.1.19 TERUMO MEDICAL CORPORATION
26.1.19.1. COMPANY OVERVIEW
26.1.19.2. REVENUE ANALYSIS
26.1.19.3. GEOGRAPHIC PRESENCE
26.1.19.4. PRODUCT PORTFOLIO
26.1.19.5. RECENT DEVELOPMENTS
26.1.20 OLYMPUS CORPORATION
26.1.20.1. COMPANY OVERVIEW
26.1.20.2. REVENUE ANALYSIS
26.1.20.3. GEOGRAPHIC PRESENCE
26.1.20.4. PRODUCT PORTFOLIO
26.1.20.5. RECENT DEVELOPMENTS
26.1.21 ZIMMER BIOMET
26.1.21.1. COMPANY OVERVIEW
26.1.21.2. REVENUE ANALYSIS
26.1.21.3. GEOGRAPHIC PRESENCE
26.1.21.4. PRODUCT PORTFOLIO
26.1.21.5. RECENT DEVELOPMENTS
26.1.22 FESENIUS MEDICAL CARE
26.1.22.1. COMPANY OVERVIEW
26.1.22.2. REVENUE ANALYSIS
26.1.22.3. GEOGRAPHIC PRESENCE
26.1.22.4. PRODUCT PORTFOLIO
26.1.22.5. RECENT DEVELOPMENTS
26.1.23 EDWARDS LIFESCIENCES CORPORATION
26.1.23.1. COMPANY OVERVIEW
26.1.23.2. REVENUE ANALYSIS
26.1.23.3. GEOGRAPHIC PRESENCE
26.1.23.4. PRODUCT PORTFOLIO
26.1.23.5. RECENT DEVELOPMENTS
26.1.24 KONINKLIJKE PHILIPS N.V.
26.1.24.1. COMPANY OVERVIEW
26.1.24.2. REVENUE ANALYSIS
26.1.24.3. GEOGRAPHIC PRESENCE
26.1.24.4. PRODUCT PORTFOLIO
26.1.24.5. RECENT DEVELOPMENTS
26.1.25 DRÄGERWERK AG & CO. KGAA
26.1.25.1. COMPANY OVERVIEW
26.1.25.2. REVENUE ANALYSIS
26.1.25.3. GEOGRAPHIC PRESENCE
26.1.25.4. PRODUCT PORTFOLIO
26.1.25.5. RECENT DEVELOPMENTS
26.1.26 COLOPLAST GROUP
26.1.26.1. COMPANY OVERVIEW
26.1.26.2. REVENUE ANALYSIS
26.1.26.3. GEOGRAPHIC PRESENCE
26.1.26.4. PRODUCT PORTFOLIO
26.1.26.5. RECENT DEVELOPMENTS
26.1.27 WATERS CORPORATION
26.1.27.1. COMPANY OVERVIEW
26.1.27.2. REVENUE ANALYSIS
26.1.27.3. GEOGRAPHIC PRESENCE
26.1.27.4. PRODUCT PORTFOLIO
26.1.27.5. RECENT DEVELOPMENTS
26.1.28 HOLOGIC, INC.
26.1.28.1. COMPANY OVERVIEW
26.1.28.2. REVENUE ANALYSIS
26.1.28.3. GEOGRAPHIC PRESENCE
26.1.28.4. PRODUCT PORTFOLIO
26.1.28.5. RECENT DEVELOPMENTS
26.1.29 STERIS
26.1.29.1. COMPANY OVERVIEW
26.1.29.2. REVENUE ANALYSIS
26.1.29.3. GEOGRAPHIC PRESENCE
26.1.29.4. PRODUCT PORTFOLIO
26.1.29.5. RECENT DEVELOPMENTS
26.1.30 INSTITUT STRAUMANN AG
26.1.30.1. COMPANY OVERVIEW
26.1.30.2. REVENUE ANALYSIS
26.1.30.3. GEOGRAPHIC PRESENCE
26.1.30.4. PRODUCT PORTFOLIO
26.1.30.5. RECENT DEVELOPMENTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.