Asia Pacific Electronic Clinical Outcome Assessment Ecoa For Content Licensed Market
Market Size in USD Million
CAGR :
% 
 USD
130.00 Million
USD
403.24 Million
2025
2033
USD
130.00 Million
USD
403.24 Million
2025
2033
| 2026 –2033 | |
| USD 130.00 Million | |
| USD 403.24 Million | |
|
|
|
|
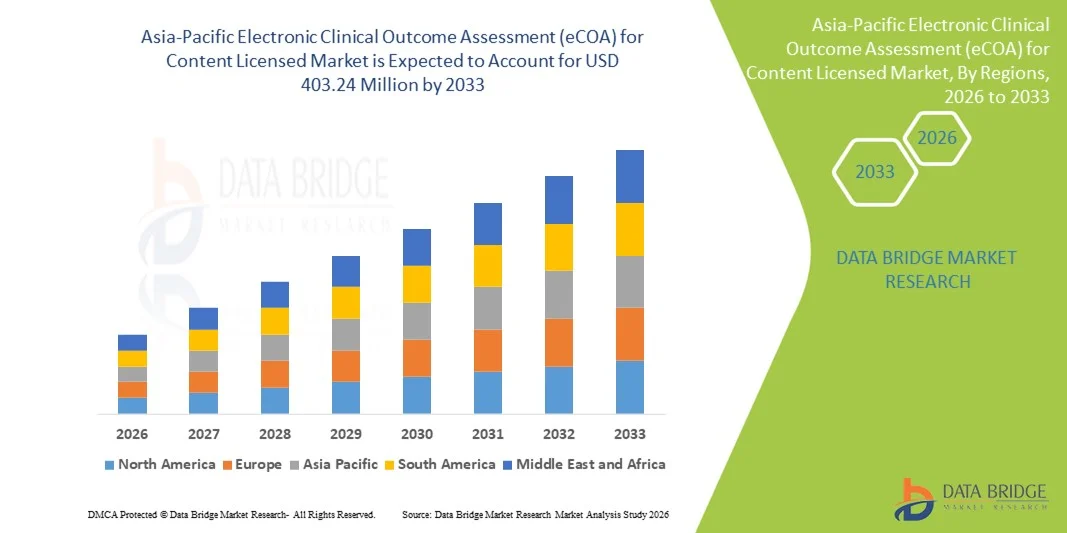
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Size
- The Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for content licensed market size was valued at USD 130.00 million in 2025 and is expected to reach USD 403.24 million by 2033, at a CAGR of 15.2% during the forecast period
- The market growth is largely fueled by increasing adoption of digital health technologies in clinical trials, regulatory encouragement for electronic data capture, and the rising demand for patient‑centric, remote outcome monitoring solutions. These trends are pushing clinical research sponsors and CROs to shift from traditional paper‑based methods to real‑time digital outcome assessment platforms
- Furthermore, rapid expansion of clinical trial activity across key Asia‑Pacific markets growing investments in cloud/ mobile eCOA platforms, and strong emphasis on data accuracy, compliance, and integration with broader eClinical systems are accelerating adoption. These converging factors are positioning eCOA as an essential component of modern clinical research infrastructure, thereby significantly boosting the industry’s growth
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Analysis
- Electronic clinical outcome assessment (eCOA) solutions, providing digital platforms for capturing patient-reported outcomes, clinician-reported outcomes, and observer-reported outcomes, are becoming essential tools in clinical trials across both pharmaceutical and medical device sectors due to their ability to improve data accuracy, enable real-time monitoring, and integrate seamlessly with broader eClinical systems
- The growing adoption of eCOA is primarily driven by the increasing focus on patient-centric clinical trials, regulatory encouragement for electronic data capture, and the rising demand for remote and decentralized trial solutions that minimize site visits while enhancing compliance and engagement
- Japan dominated the Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for content licensed market in 2025 with the largest revenue share of 38.5%, characterized by advanced digital healthcare infrastructure, a high number of ongoing clinical trials, and the presence of leading technology providers offering integrated eCOA platforms
- China is expected to be the fastest-growing country in the market during the forecast period due to increasing clinical trial activity, growing investment in mobile and cloud-based platforms, expanding regulatory support for electronic data capture, and rising adoption of patient-centric digital solutions
- Patient-reported outcome (PRO) segment dominated the Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for content licensed market with a market share of 45.2% in 2025, driven by its critical role in capturing real-time patient feedback, ensuring compliance with regulatory standards, and providing actionable insights for trial sponsors
Report Scope and Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Segmentation
|
Attributes |
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Trends
Digital and Mobile Integration for Patient-Centric Trials
- A significant and accelerating trend in the Asia-Pacific eCOA for content licensed market is the integration of mobile devices, cloud platforms, and patient-facing apps, enabling real-time collection of clinical outcomes while enhancing patient engagement and trial adherence
- For instance, platforms such as Medidata eCOA Mobile allow patients to submit PRO and clinician-reported outcomes via smartphones and tablets, enabling seamless remote data capture and improved compliance
- Integration of digital and mobile platforms with electronic health records (EHR) and broader eClinical systems enables real-time data monitoring, error reduction, and streamlined regulatory reporting. For instance, this allows sponsors to track patient responses instantly and adjust protocols as needed
- The growing adoption of wearable devices and sensors for continuous patient monitoring complements eCOA platforms, allowing collection of high-frequency, objective data alongside subjective outcomes for a more holistic view of patient health
- This trend towards more integrated, patient-friendly, and real-time digital outcome assessment is fundamentally reshaping clinical trial processes. For instance, companies such as CRF Health and ERT are developing mobile-enabled eCOA solutions with customizable interfaces and automated reminders to enhance patient compliance
- The demand for digital, mobile, and cloud-integrated eCOA platforms is rapidly growing across both pharmaceutical and medical device trials, as sponsors prioritize efficient, accurate, and patient-centric data collection
- The use of multilingual and culturally adapted eCOA interfaces is becoming more common to support diverse patient populations across Asia-Pacific countries. For instance, platforms now include local languages and culturally sensitive question formats to improve compliance and data quality
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Dynamics
Driver
Rising Demand for Patient-Centric and Remote Clinical Trials
- The increasing focus on patient-centric trials, coupled with the shift toward decentralized and hybrid clinical trials, is a key driver of the Asia-Pacific eCOA for content licensed market
- For instance, in 2025, Medrio launched an eCOA module designed for remote trials in India and China, enabling sponsors to capture outcomes without requiring frequent site visits
- As trial sponsors aim to improve patient engagement and compliance, eCOA platforms provide real-time monitoring, alerts, and reminders, which reduce missing data and enhance overall trial quality
- Furthermore, the growing adoption of digital and mobile technologies across clinical research sites is making eCOA solutions a preferred choice, offering seamless integration with other eClinical tools and data systems
- The ability to remotely capture patient-reported outcomes, clinician-reported outcomes, and observer-reported outcomes through smartphones or tablets is a key factor accelerating adoption in both pharmaceutical and medical device trials
- The expansion of hybrid and decentralized trials in Asia-Pacific, combined with increasing regulatory encouragement for electronic data capture, further supports the robust growth of eCOA solutions
- Rising investment from multinational pharmaceutical companies in Asia-Pacific clinical trials is increasing demand for scalable and standardized eCOA platforms. For instance, sponsors are deploying unified platforms across multiple countries to improve efficiency and compliance
- Growing emphasis on data-driven decision-making and faster clinical trial timelines is encouraging sponsors to adopt eCOA solutions that provide instant analytics and reporting. For instance, real-time dashboards allow trial managers to monitor patient responses and adjust protocols proactively
Restraint/Challenge
Data Privacy Concerns and Regulatory Compliance Hurdles
- Concerns surrounding patient data security, privacy, and regulatory compliance pose significant challenges for broader adoption of eCOA platforms in Asia-Pacific
- For instance, high-profile reports of electronic health data breaches have made some sponsors and patients cautious about remote digital data collection
- Ensuring compliance with regional regulations, such as Japan’s Act on the Protection of Personal Information (APPI) and China’s Personal Information Protection Law (PIPL), requires robust encryption, secure authentication, and validated software, which can be complex and costly to implement
- Furthermore, differences in regulatory standards and requirements across countries in Asia-Pacific can slow platform deployment and adoption, requiring additional localization and validation efforts
- While cloud and mobile eCOA platforms improve convenience and efficiency, the perceived risk of cybersecurity breaches and non-compliance can hinder adoption, particularly among smaller sponsors or CROs with limited IT resources
- Overcoming these challenges through strong data security measures, regulatory guidance adherence, and clear communication of compliance practices will be vital for sustained market growth
- Limited technical expertise and infrastructure at smaller clinical sites in certain Asia-Pacific countries can slow adoption of advanced eCOA solutions. For instance, sites may lack the IT support to implement cloud-based or mobile eCOA systems efficiently
- Variability in patient digital literacy can also be a barrier, as some participants may struggle with mobile or web-based platforms. For instance, sponsors need to provide training and intuitive interfaces to ensure accurate and complete data collection
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Scope
The market is segmented on the basis of product, approach, end user, and platform.
- By Product
On the basis of product, the Asia-Pacific eCOA for content licensed market is segmented into On-Premise Solutions, Cloud-Based Solutions, and Web-Based Solutions. Cloud-Based Solutions dominated the market with the largest revenue share in 2025, driven by their flexibility, scalability, and ability to support remote clinical trials across multiple sites. Sponsors increasingly prefer cloud platforms as they enable real-time access to patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), and other eCOA data without the need for extensive IT infrastructure at individual sites. Cloud-based solutions also facilitate integration with other eClinical systems, reducing data discrepancies and accelerating analysis. Their adoption is further supported by regulatory acceptance for electronic data capture in decentralized trials. The ability to support mobile devices, tablets, and wearables enhances patient engagement and compliance. Cloud solutions also allow seamless software updates and centralized management of trial data, reducing operational costs for sponsors.
Web-Based Solutions are anticipated to witness the fastest growth from 2026 to 2033, fueled by the increasing need for browser-accessible platforms that do not require installation of heavy software. Web-based eCOA tools enable clinical sites and patients to enter data from any device with an internet connection, improving participation in decentralized trials. They are particularly attractive for academic institutions and smaller research organizations that seek cost-effective, scalable solutions. Web-based solutions also support multilingual and culturally adapted interfaces, critical for trials spanning multiple Asia-Pacific countries. Ease of use, low maintenance requirements, and compatibility with various operating systems are key drivers of their adoption. The growing preference for hybrid trials that combine site visits with remote data capture further accelerates web-based solution uptake.
- By Approach
On the basis of approach, the market is segmented into Clinician-Reported Outcome Assessment (ClinRO), Patient-Reported Outcome Assessment (PRO), Observer-Reported Outcome Assessment (ObsRO), and Performance Outcome Assessment (PerfO). Patient-Reported Outcome Assessment (PRO) dominated the market with the largest revenue share of 45.2% in 2025, driven by the increasing emphasis on patient-centric clinical trials. PRO platforms allow patients to directly report their symptoms, quality of life, and treatment responses, providing sponsors with authentic, real-time insights. The adoption of mobile and cloud-enabled PRO platforms has enhanced compliance and reduced missing data, particularly in decentralized or hybrid trials. Regulatory agencies, including Japan’s PMDA and China’s NMPA, increasingly encourage electronic PRO data collection for accurate endpoints. PRO solutions also enable real-time monitoring and automated alerts, allowing clinical teams to respond quickly to adverse events or noncompliance. Their dominance is supported by increasing demand in therapeutic areas such as oncology, neurology, and chronic diseases where patient feedback is critical.
Observer-Reported Outcome Assessment (ObsRO) is expected to witness the fastest growth from 2026 to 2033, particularly in pediatric, geriatric, and rare disease trials. ObsRO platforms allow caregivers or study staff to report patient outcomes in cases where patients cannot self-report, ensuring comprehensive and accurate data collection. The rise of remote monitoring and mobile-enabled platforms has increased ObsRO adoption, making it easier for observers to submit data in real time. ObsRO solutions are also gaining traction due to regulatory emphasis on including vulnerable patient populations in trials. The growing complexity of clinical studies and the need for multi-source data validation further drive ObsRO uptake. Integration with other eCOA approaches, such as PRO and ClinRO, enhances data quality and provides holistic insights for sponsors.
- By End User
On the basis of end user, the market is segmented into commercial service providers, hospitals and transplant centers, research laboratories, and academic institutions. Commercial Service Providers dominated the market with the largest revenue share in 2025, driven by their expertise in providing scalable, validated eCOA solutions for multiple sponsors and trials. These providers offer end-to-end services including software deployment, training, technical support, and regulatory compliance guidance. Their dominance is further supported by the growing outsourcing trend among pharmaceutical and biotech companies, which prefer commercial providers to manage complex decentralized trials. Cloud-based deployment and real-time monitoring services enhance efficiency and patient compliance. Commercial service providers also offer customizable solutions tailored to therapeutic areas, study size, and regional regulations, making them the preferred choice for multinational trials. Their ability to integrate with other eClinical platforms ensures centralized data management and faster trial timelines.
Academic Institutions are expected to witness the fastest growth from 2026 to 2033, fueled by increasing clinical research activity in universities and teaching hospitals across Asia-Pacific. These institutions require cost-effective, flexible eCOA solutions that support research protocols, patient engagement, and regulatory compliance. The adoption of web-based and mobile-enabled eCOA platforms facilitates participation in multicenter trials and collaborative studies. Academic end users also benefit from platforms that support data anonymization and secure sharing for research purposes. Increased government funding and initiatives to digitize clinical research in countries such as India, China, and Japan are further accelerating adoption. The need for real-time reporting and outcome analysis for academic publications also drives growth in this segment.
- By Platform
On the basis of platform, the market is segmented into contract research organizations, pharmaceutical and biopharmaceutical companies, medical device manufacturers, hospitals and clinical laboratories, consulting service companies, research and academia, and others. Contract Research Organizations (CROs) dominated the market with the largest revenue share in 2025, driven by their capability to manage multiple clinical trials across diverse geographies using centralized, validated eCOA systems. CROs provide sponsors with the advantage of outsourcing technical and regulatory complexities, including system setup, training, and compliance audits. Their cloud-based platforms allow real-time access to clinical outcome data, improving monitoring and accelerating decision-making. CROs also leverage AI-enabled analytics to identify trends, monitor patient compliance, and flag inconsistencies. Their dominance is reinforced by strong partnerships with pharmaceutical and biotech companies conducting large-scale trials in Asia-Pacific. Standardized eCOA platforms across CROs also facilitate multi-country regulatory submissions.
Pharmaceutical and Biopharmaceutical Companies are expected to witness the fastest growth from 2026 to 2033, as these companies increasingly adopt in-house eCOA platforms to streamline clinical trials and improve patient-centric data capture. Direct deployment enables sponsors to maintain control over data integrity and compliance while reducing reliance on third-party service providers. Growth is fueled by increasing investment in mobile and cloud-based solutions that support hybrid and decentralized trial models. Companies are also leveraging eCOA platforms to accelerate trial timelines, enhance patient engagement, and comply with regulatory requirements for electronic data collection. Integration with other digital systems, such as EHR and laboratory data management, further strengthens the adoption among pharmaceutical and biotech companies.
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Regional Analysis
- Japan dominated the Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for content licensed market in 2025 with the largest revenue share of 38.5%, characterized by advanced digital healthcare infrastructure, a high number of ongoing clinical trials, and the presence of leading technology providers offering integrated eCOA platforms
- Sponsors and clinical sites in Japan highly value the efficiency, real-time monitoring, and regulatory-compliant features offered by eCOA platforms, enabling accurate and timely capture of patient, clinician, and observer-reported outcomes
- This widespread adoption is further supported by well-established clinical research networks, increasing government support for digital health technologies, and growing interest from pharmaceutical and biopharmaceutical companies in decentralized and hybrid trial models, establishing eCOA as a preferred solution for clinical outcome capture
Japan Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Insight
The Japan Electronic Clinical Outcome Assessment (eCOA) for content licensed market is gaining momentum due to the country’s advanced digital healthcare infrastructure, high clinical trial activity, and focus on patient-centric research. The adoption of mobile-enabled PRO and ClinRO platforms is driven by the increasing number of smart hospitals and connected clinical research sites. Integration with EHR systems and broader eClinical platforms is improving real-time monitoring, regulatory compliance, and operational efficiency. Japan’s aging population is also driving demand for patient-friendly and observer-reported outcome (ObsRO) solutions in both residential and hospital-based trials. Furthermore, sponsors are leveraging AI and analytics in eCOA platforms to enhance data quality and optimize trial decision-making.
China Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Insight
The China Electronic Clinical Outcome Assessment (eCOA) for content licensed market is expected to grow at the fastest rate in the Asia-Pacific region during the forecast period, fueled by a surge in clinical trials, government incentives for digital health adoption, and increasing use of mobile and cloud-based outcome assessment platforms. Sponsors are increasingly implementing decentralized trials to reach broader patient populations, leveraging eCOA solutions for real-time patient monitoring. Integration with wearable devices and remote reporting tools is enhancing data accuracy and compliance. The growth is also supported by expanding CRO services and domestic platform providers offering scalable, cost-effective solutions. In addition, regulatory acceptance for electronic PRO, ClinRO, and ObsRO data is encouraging adoption among pharmaceutical and biotech companies.
India Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Insight
The India Electronic Clinical Outcome Assessment (eCOA) for content licensed market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to the country’s growing clinical research outsourcing industry, increasing digital literacy among patients, and rapid adoption of mobile health technologies. The rise of decentralized trials, coupled with affordable and scalable cloud-based eCOA platforms, is facilitating broader adoption in hospitals, academic institutions, and CROs. India’s expanding clinical trial ecosystem, supported by regulatory encouragement for electronic outcome capture, is enabling faster data collection and improved patient compliance. Furthermore, multinational sponsors are increasingly selecting India for multi-country studies due to cost efficiency and availability of skilled personnel. The adoption of patient-centric PRO and ObsRO solutions is further enhancing trial quality and real-time monitoring capabilities.
Australia Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Insight
The Australia Electronic Clinical Outcome Assessment (eCOA) for content licensed market is witnessing steady growth due to the country’s advanced healthcare infrastructure, high adoption of digital health technologies, and increasing number of clinical trials conducted in hospitals and research institutions. Sponsors and CROs value Australia for its regulatory alignment with global standards, making it easier to implement electronic PRO, ClinRO, ObsRO, and PerfO platforms. The widespread use of mobile and web-based eCOA solutions enhances patient engagement and compliance, particularly in decentralized or hybrid trials. Integration with electronic health records (EHR) and other digital systems improves data accuracy and streamlines trial management. Furthermore, Australia’s skilled clinical workforce and emphasis on patient-centric research drive the adoption of innovative eCOA platforms across pharmaceutical, biopharmaceutical, and medical device trials.
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Share
The Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed industry is primarily led by well-established companies, including:
- Veeva Systems (U.S.)
- Kayentis (France)
- Signant Health (U.S.)
- IQVIA Holdings, Inc. (U.S.)
- Clinical Ink, Inc. (U.S.)
- Medidata Solutions (U.S.)
- eCOA GmbH (Germany)
- CRF Health (U.K.)
- Motentia, LLC (U.S.)
- Bracket Global LLC (U.S.)
- SHYFT Analytics (U.S.)
- Mednet Solutions (Australia)
- SureClinical (U.S.)
- Relypsa (U.S.)
- OpenClinica, LLC (U.S.)
- Clario (U.S.)
- Datacubed Health (U.S.)
- Florence Healthcare (U.S.)
- bloqcube (U.S.)
- Realtime Clinical (U.S.)
What are the Recent Developments in Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market?
- In March 2024, major clinical research service providers such as PPD (Thermo Fisher Scientific) publicly highlighted their digital and decentralized trial ecosystems, emphasizing tools and methodologies that support digital outcome capture including eCOA designed to improve data quality, reduce patient burden, and increase trial participation across regions including Asia‑Pacific
- In December 2023, ObvioHealth announced the launch of its innovative electronic clinical outcome assessment (eCOA) solution integrated into its ObvioGo® platform, enabling rapid eCOA creation, enhanced patient engagement, and up to 70% faster implementation timelines for clinical trials
- In August 2023, China’s National Medical Products Administration (NMPA) and the Center for Drug Evaluation (CDE) released the Technical Guidelines for the Implementation of Patient‑Centered Drug Clinical Trials, formally recognizing electronic tools such as eCOA, eConsent, and remote data collection to modernize clinical trial practices. This regulatory clarity supports expanded use of eCOA technologies in Asia‑Pacific trials
- In June 2023, ICON plc announced a major update to its ICON Digital Platform, which includes an integrated eCOA module pre‑loaded with validated assessment libraries. The platform supports patient engagement, eConsent, eCOA, and remote data capture across traditional and decentralized trials, reducing setup time and improving outcome data consistency
- In April 2023, Almac Clinical Technologies announced the launch of its IXRS®3 Partnership Network, enabling biopharmaceutical sponsors and CROs to share data and interoperable clinical technologies including eCOA modules to create more seamless and inclusive trial experiences for sites and patients. This initiative emphasizes interoperability and collaboration to streamline trial data capture and enhance eCOA integration across multi‑site studies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.