Middle East And Africa Adalimumab Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
134.81 Million
USD
181.68 Million
2021
2029
USD
134.81 Million
USD
181.68 Million
2021
2029
| 2022 –2029 | |
| USD 134.81 Million | |
| USD 181.68 Million | |
|
|
|
Middle East and Africa Adalimumab Market, By Drug class (Antirheumatics, TNF Alfa Inhibitors, Others), Indication (Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Plaque Psoriasis, Crohn's Disease, Ulcerative Colitis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Hidradenitis Suppurativa, Non-Infectious Intermediate, Others), Type (Biologics, Biosimilars), Dosage Strength (40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, Others), Drug Type (Branded, Generics), Route of Administration (Oral, Parenteral, Others), Age Group (Pediatric, Adult, Geriatric), Dosage Form (Tablet, Injection, Solution, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Market Analysis and Size
Adalimumab, which was first licensed in the United States, is now available in more than 60 countries. Its Middle East and Africa market is consolidated, with only a few firms attempting to outsmart one other on price. Most of the major players are currently concentrating their efforts on the development of adalimumab biosimilars for the treatment of rheumatoid arthritis and psoriasis. This is seen in clinical trials testing the safety and efficacy of adalimumab biosimilars in the treatment of medical disorders. Many inflammatory disorders in adults are treated with adalimumab, including ulcerative colitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, and hidradenitis suppurativa.
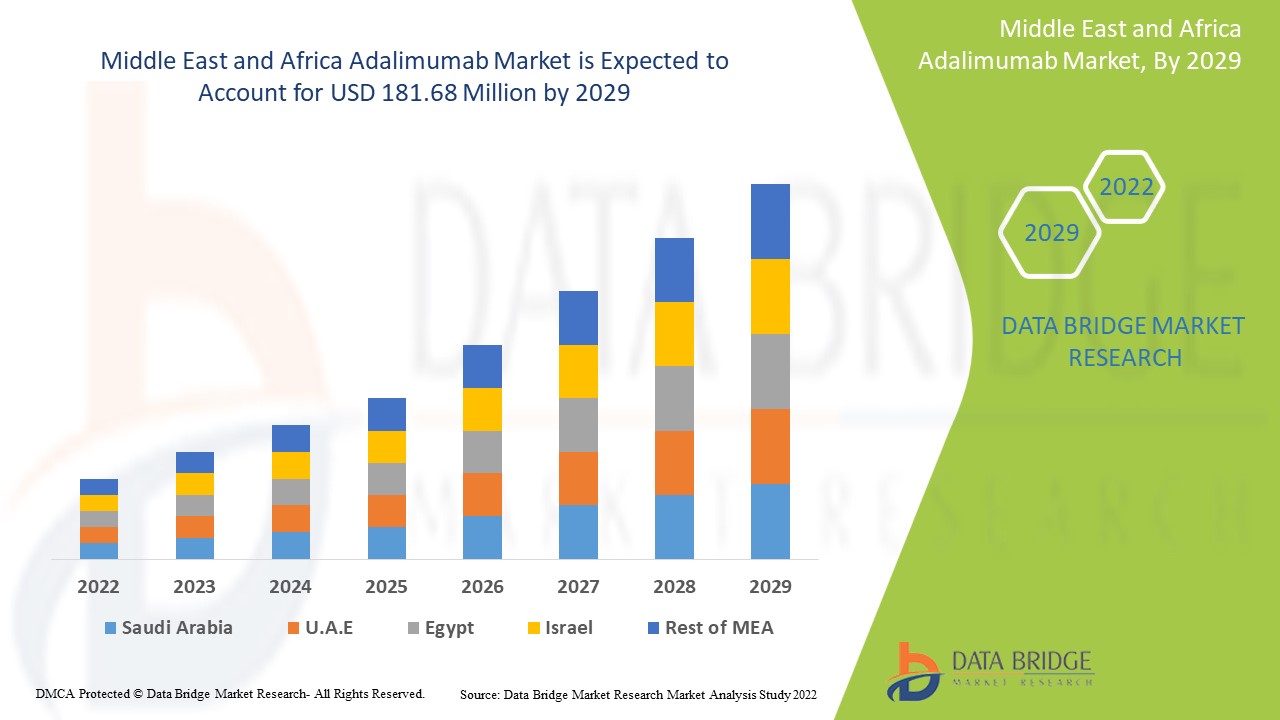
Data Bridge Market Research analyses that the Middle East and Africa adalimumab market was valued at USD 134.81 million in 2021 and is expected to reach USD 181.68 million by 2029, registering a CAGR of 3.80% during the forecast period of 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Drug class (Antirheumatics, TNF Alfa Inhibitors, Others), Indication (Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Plaque Psoriasis, Crohn's Disease, Ulcerative Colitis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Hidradenitis Suppurativa, Non-Infectious Intermediate, Others), Type (Biologics, Biosimilars), Dosage Strength (40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, Others), Drug Type (Branded, Generics), Route of Administration (Oral, Parenteral, Others), Age Group (Pediatric, Adult, Geriatric), Dosage Form (Tablet, Injection, Solution, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) |
|
Countries Covered |
Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA). |
|
Market Players Covered |
Mylan N.V. (US), AbbVie Inc. (US), Zydus Cadila (India), Pfizer Inc. (US), Hetero Biopharma Ltd. (India), Boehringer Ingelheim International GmbH. (Germany) |
|
Market Opportunities |
|
Market Definition
Adalimumab is a prescription medicine which is sold under the brand names Humira and Exemptia. heumatoid arthritis, psoriatic arthritis, Crohn's disease, psoriasis, and ulcerative colitis are all treated with adalimumab. TNF (tumour necrosis factor alpha) is commonly bound by adalimumab. When TNF interacts to TBF receptors, an inflammatory response to autoimmune illness is triggered. By binding to a TNF, adalimumab reduces the likelihood of an inflammatory response.
Middle East and Africa Adalimumab Market Dynamics
Drivers
- Rise in the incidence rate of autoimmune disease
The rise in incidences of autoimmune disease such as psoriatic arthritis, plaque psoriasis, ulcerative colitis, ankylosing spondylitis, rheumatoid arthritis and Crohn's disease is anticipated to flourish the growth rate of the market. Adalimumab is a medication that reduces pain and swelling while also slowing the progression of arthritis. Adalimumab is used to treat active enthesitis-related arthritis, Rheumatoid arthritis, osteoarthritis, polyarticular juvenile idiopathic arthritis and other autoimmune conditions. Along with this, the growing prevalence of chronic disorders will enhance the demand of adalimumab market.
- Increasing investment for healthcare infrastructure
Another significant factor influencing the growth rate of adalimumab market is the rising healthcare expenditure which helps in improving its infrastructure. Also, various government organizations aims to improve the healthcare infrastructure by increasing funding and this will further influence the market dynamics.
- Rising incidences of skin disorders
The incidences of skin disorders is estimated to propel the market’s growth rate during the forecast period of 2022-2029. The World Health Organization (WHO) estimates that 900 million people worldwide suffer from skin ailments at any given moment. TNF-alpha (tumor necrosis factor-alpha) is a critical participant in the inflammatory process that causes skin disorders including psoriasis. Adalimumab targets this protein in the body. Psoriasis is a skin condition that develops scaly red patches on the knees, elbows, trunk, and scalp. Psoriasis is caused by an overactive immune system response, which is suppressed by adalimumab. According to the National Psoriasis Foundation, 125 million individuals worldwide have psoriasis, accounting for 2 to 3 percent of the overall population, fueling market growth.
Furthermore, rising initiatives by public and private organizations to spread awareness and surging demand for biosimilar drugs owing to their cost-effectiveness will expand the adalimumab market. Additionally, surging number of geriatric population and rising cases of upper respiratory tract infection will result in the expansion of adalimumab market.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by an increase in the number of research and development activities. This will provide beneficial opportunities for the adalimumab market growth. Along with this, rising drug approvals and launches will further propel the market’s growth rate.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the adalimumab market growth during the forecast period.
Restraints/Challenges
- High cost as well as side effects associated with adalimumab
Adalimumab is quite expensive for persons in low and middle-income nations, costing roughly USD 2000-3000 each infusion. Furthermore, adalimumab's negative effects are expected to limit market expansion. Fever, swollen glands, night sweats, general sensation of unwell, joint and muscle pain, skin rash, easy bruising or bleeding, and others are some of the frequent adverse effects of adalimumab. Adalimumab can also cause a type of lymphoma that is fatal, as well as cancers of the liver, spleen, and bone marrow. This is most common in teenagers and young men with Crohn's disease or ulcerative colitis, which slows market growth.
On the other hand, the lack of healthcare infrastructure in developing economies and strict regulatory process linked with product approval of biosimilars will challenge the adalimumab market. Additionally, patent expiration of drugs will act as restrain and further impede the growth rate of market during the forecast period of 2022-2029.
This Middle East and Africa adalimumab market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Middle East and Africa adalimumab market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
Middle East and Africa adalimumab market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
COVID-19 Impact on Middle East and Africa Adalimumab Market
Since its emergence in December 2019, the COVID-19 virus has spread to nearly every country on the planet, prompting the World Health Organization (WHO) to declare it a public health emergency. COVID-19, a new coronavirus, was identified as the causal agent in the pneumonia cases. This virus spread quickly over the world, killing a large number of people. COVID-19 was labelled a Middle East and Africa pandemic by the World Health Organization (WHO) in March 2020, and rigorous measures to prevent the disease's spread were recommended. Since then, the pandemic had delayed the expansion of the healthcare sector and disrupted the supply chain. Furthermore, governments in a number of nations have imposed nationwide lockdowns in order to halt the spread of COVID-19. Similarly, healthcare organizations in numerous nations throughout the world were having difficulty continuing their supply chain activities. The adalimumab market was hampered by the supply chain slowness.
Recent Development
- In October 2021, The U.S. Food and Drug Administration (FDA) had announced the approval of first interchangeable biosimilar product for the treatment of various inflammatory diseases. The biosimilar and interchangeable approval pathway was established to help patients with critical medical conditions gain access to more treatment options. Cyltezo is the first interchangeable monoclonal antibody and the second interchangeable biosimilar medicine authorized by the FDA.
Middle East and Africa Adalimumab Market Scope
The Middle East and Africa adalimumab market is segmented on the basis of drug class, type, indication, dosage form, dosage strength, drug type, route of administration, age group, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug class
- Antirheumatics
- TNF Alfa Inhibitors
- Others
Indication
- Rheumatoid Arthritis
- Ankylosing Spondylitis
- Chronic Plaque Psoriasis
- Crohn's Disease
- Ulcerative Colitis
- Psoriatic Arthritis
- Juvenile Idiopathic Arthritis
- Hidradenitis Suppurativa
- Non-Infectious Intermediate
- Others
Type
- Biologics
- Biosimilars
Dosage Strength
- 40mg/0.4mlg
- 80mg/0.8mlg
- 20mg/0.2mlg
- 10mg/0.1mlg
- Others
Drug Type
- Branded
- Generics
Route of Administration
- Oral
- Parenteral
- Others
Dosage Form
- Injection
- Solution
- Tablet
- Others
Age Group
- Pediatric
- Adult
- Geriatric
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Middle East and Africa Adalimumab Market Regional Analysis/Insights
The Middle East and Africa adalimumab market is analysed and market size insights and trends are provided by country, drug class, type, indication, dosage form, dosage strength, drug type, route of administration, age group, end-users and distribution channel as referenced above.
The countries covered in the Middle East and Africa adalimumab market report are Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA).
Saudi Arabia dominates the adalimumab market because of the growing number of research and development activities to overcome the burden of arthritis diseases in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Middle East and Africa brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East and Africa Adalimumab Market Share Analysis
The Middle East and Africa adalimumab market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Middle East and Africa presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Middle East and Africa adalimumab market.
Some of the major players operating in the Middle East and Africa adalimumab market are:
- Mylan N.V. (US)
- Zydus Cadila (India)
- Boehringer Ingelheim International GmbH. (Germany)
- AbbVie Inc. (US)
- Abbott (US)
- Hetero Biopharma Ltd. (India)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2 MG/0.4ML
13.3 MG/0.8ML
13.4 MG/0.4ML
13.5 MG/0.1ML
13.6 OTHERS
14 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 MIDDLE EAST & AFRICA
19.1.1 SAUDI ARABIA
19.1.2 SOUTH AFRICA
19.1.3 UAE
19.1.4 ISRAEL
19.1.5 KUWAIT
19.1.6 EGYPT
19.1.7 REST OF MIDDLE EAST & AFRICA
20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
Lista de Tabela
LIST OF TABLES
TABLE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 SAUDI ARABIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 SAUDI ARABIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 SAUDI ARABIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 SAUDI ARABIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 SAUDI ARABIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 SAUDI ARABIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 SAUDI ARABIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 SAUDI ARABIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 SAUDI ARABIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 SAUDI ARABIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 SOUTH AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 SOUTH AFRICA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 SOUTH AFRICA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 SOUTH AFRICA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 SOUTH AFRICA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 SOUTH AFRICA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 SOUTH AFRICA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 SOUTH AFRICA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 SOUTH AFRICA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 SOUTH AFRICA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 UAE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 UAE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 UAE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 UAE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 UAE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 UAE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 UAE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 UAE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 UAE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 UAE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 ISRAEL ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 ISRAEL ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 ISRAEL BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 ISRAEL ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 ISRAEL ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 ISRAEL GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 ISRAEL ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 ISRAEL ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 ISRAEL ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 ISRAEL ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 KUWAIT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 KUWAIT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 KUWAITBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 KUWAIT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 KUWAIT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 KUWAIT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 KUWAIT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 KUWAIT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 KUWAIT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 KUWAIT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 EGYPT ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 EGYPT ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 EGYPT BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 EGYPT ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 EGYPT ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 EGYPT GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 EGYPT ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 EGYPT ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 EGYPT ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 EGYPT ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 REST OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
Lista de Figura
LIST OF FIGURES
FIGURE 1 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF MIDDLE EAST & AFRICA ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 MIDDLE EAST & AFRICA ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.