Mercado global de testes de cancro hereditário, por tipo de teste (conjunto de vários painéis e teste genético de local único), tipo de diagnóstico (biópsia, imagiologia, testes laboratoriais), tecnologia (sequenciação, reação em cadeia da polimerase (PCR) , microarray), tipo de doença (hereditária Síndrome do Cancro da Mama e do Ovário, Síndrome de Cowden, Síndrome de Lynch, Síndromes de Leucemia Hereditária e Malignidades Hematológicas, Polipose Adenomatosa Familiar (FAP), Síndrome de Li-Fraumeni, Doença de Von Hippel -Lindau, Síndromes de Neoplasias Endócrinas Múltiplas (MEN)), Utilizador Final (Hospitais , Clínicas, Laboratórios, Centros de Radiologia, Centros de Diagnóstico, Outros), Canal de Distribuição (Licitação Directa, Vendas a Retalho), Tendências do Sector e Previsão para 2029.
Análise e Insights do Mercado de Testes de Cancro Hereditário
O cancro é uma doença genética causada por determinadas mutações nos genes que controlam a função das células, afetando particularmente o seu crescimento e reprodução. As mutações genéticas herdadas são responsáveis por aproximadamente 5-10% de todos os cancros. Os investigadores associaram mutações em genes específicos a mais de 50 síndromes hereditários de cancro que afetam as pessoas através do desenvolvimento de certos tipos de cancro. Além disso, cerca de 5 a 10% dos casos de cancro da mama estão associados a mutações genéticas herdadas dos pais. Desta forma, a crescente prevalência do cancro está a impulsionar o crescimento constante dos cancros hereditários e, consequentemente, o crescimento do mercado dos testes hereditários de cancro. Além disso, a procura crescente por métodos de teste não invasivos e a procura crescente por cuidados de saúde de melhor qualidade e diagnóstico precoce são as principais oportunidades para o crescimento do mercado. Além disso, os desafios éticos enfrentados durante os testes hereditários de cancro e a crescente concorrência entre os participantes do mercado são os principais desafios para o crescimento do mercado.
No entanto, as regulamentações rigorosas de diagnóstico de cancro e o elevado custo associado aos testes podem dificultar o crescimento do mercado.
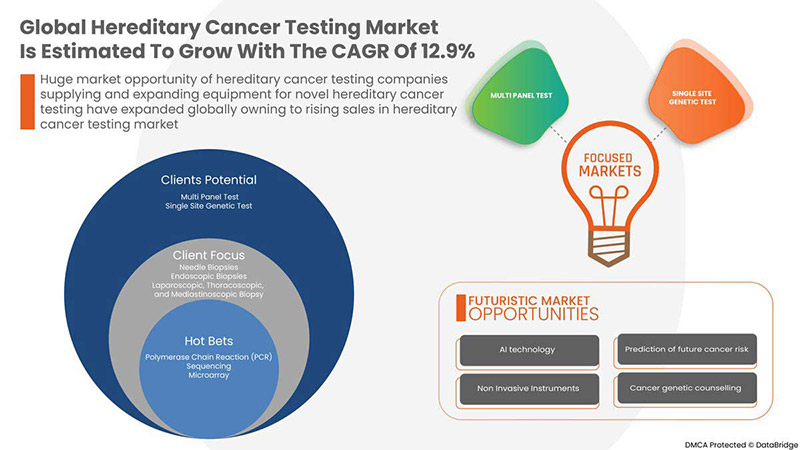
A Data Bridge Market Research analisa que o mercado global de testes de cancro hereditário deverá atingir o valor de 13.085,04 milhões de dólares até 2029, com um CAGR de 12,9% durante o período previsto. Este relatório de mercado abrange também a análise de preços, a análise de patentes e os avanços tecnológicos em profundidade.
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2022 a 2029 |
|
Ano base |
2021 |
|
Anos históricos |
2020 (Personalizável para 2019 - 2015) |
|
Unidades quantitativas |
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos |
|
Segmentos abrangidos |
Por tipo de teste (conjunto de painéis múltiplos e teste genético de sítio único), tipo de diagnóstico (biópsia, imagiologia, testes laboratoriais), tecnologia (sequenciação, reação em cadeia da polimerase (PCR), microarray), tipo de doença ( síndrome hereditário do cancro da mama e do ovário, Síndrome de Cowden, Síndrome de Lynch, Síndromes de Leucemia Hereditária e Malignidades Hematológicas, Polipose Adenomatosa Familiar (FAP), Síndrome de Li-Fraumeni, Doença de Von Hippel-Lindau, Síndromes de Neoplasias Endócrinas Múltiplas (MEN)), Utilizador Final (Hospitais, Clínicas, Laboratórios, Centros de Radiologia, Centros de Diagnóstico, Outros), Canal de Distribuição (Concurso Directo, Venda a Retalho. |
|
Países abrangidos |
EUA, Canadá, México, Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Turquia, Bélgica, Holanda, Suíça e restante Europa, China, Japão, Índia, Coreia do Sul, Singapura, Tailândia, Malásia, Austrália, Filipinas , Indonésia e restante Ásia-Pacífico, África do Sul, Arábia Saudita, Emirados Árabes Unidos, Egito, Israel e restante Médio Oriente e África, Brasil, Argentina e restante América do Sul. |
|
Atores do mercado abrangidos |
Invitae Corporation, Illumina, Inc., Natera, Inc., CENTOGENE NV, 4baseCare, Biocartis, Fulgent Genetics, Ambry Genetics, BioReference, PerkinElmer Inc., LifeLabs, Abbott, BIO-HELIX, Cepheid, Eurofins Scientific, entre outros. |
Definição do mercado global de testes de cancro hereditário
O cancro hereditário é qualquer cancro causado por uma mutação genética herdada. Variantes prejudiciais em certos genes estão associadas a um risco aumentado de cancro. Os testes genéticos podem estimar o risco de uma pessoa desenvolver cancro ao longo da vida. Isto pode ser feito procurando mutações nos seus genes, cromossomas ou proteínas. Os testes genéticos estão disponíveis para vários tipos de cancro. Isto inclui cancro da mama, ovário, cólon, tiroide, próstata, pâncreas, pele, sarcoma, rim e estômago. Numerosos estudos médicos mostram que 5% a 10% dos cancros comuns são considerados hereditários. Os testes genéticos são realizados para determinar se uma pessoa é portadora de uma variante genética prejudicial. Estes testes também ajudam a determinar se um membro da família que ainda não teve cancro herdou a mesma variante de um membro da família conhecido por ter uma alternativa de suscetibilidade ao cancro.
Dinâmica do mercado global de testes de cancro hereditário
Esta secção trata da compreensão dos impulsionadores, vantagens, oportunidades, restrições e desafios do mercado. Tudo isto é discutido em detalhe abaixo:
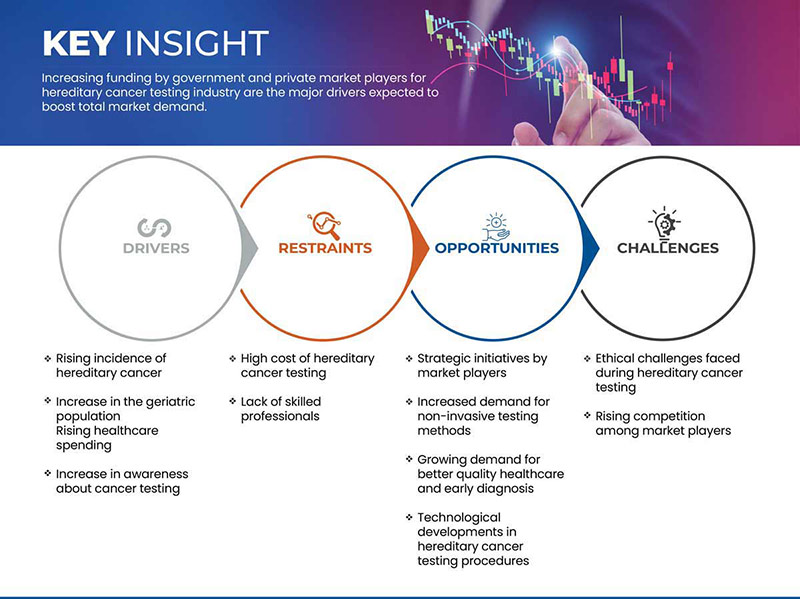
Motoristas
- Aumento da incidência de cancro hereditário
O cancro surge do crescimento descontrolado de células. O cancro é causado por alterações prejudiciais (mutações) nas mensagens genéticas (genes) que controlam o crescimento e a divisão das células, impedindo-as de fazer o seu trabalho de forma eficaz.
Nos casos de cancro hereditário, o indivíduo herda uma cópia do gene regulador do crescimento mutado de um dos progenitores e uma cópia funcional do mesmo gene do outro. O gene mutado é também chamado de "gene de suscetibilidade ao cancro". Como este gene de suscetibilidade ao cancro é herdado, encontra-se em todas as células do corpo, mas uma cópia funcional do gene mantém cada célula a funcionar corretamente. No entanto, se uma mutação danificar uma cópia funcional de um gene numa célula, esta pode perder o controlo do seu crescimento e tornar-se cancerígena. Assim, os indivíduos que herdam um gene de cancro têm muito maior probabilidade de desenvolver determinados tipos de cancro durante a sua vida.
Assim, o aumento da incidência de cancro hereditário aumenta a procura de testes de cancro hereditário e pode atuar como um impulsionador do crescimento do mercado global de testes de cancro hereditário.
- Aumento da população geriátrica
O cancro pode ser uma doença de doentes idosos. A população geriátrica está a aumentar em todo o mundo. O risco de cancro hereditário entre os idosos é muito maior. O aumento da população geriátrica pode resultar num melhor serviço ao mercado global de testes de cancro hereditário. Antecipou um aumento da procura no mercado global de testes de cancro hereditário. O envelhecimento da população está a provocar uma redistribuição da estrutura demográfica que irá afectar o futuro dos cuidados de saúde. Sem dúvida, o risco de cancro aumenta exponencialmente com a idade.
O cancro hereditário, incluindo a sua incidência e risco associado, utilizando a maior base de dados de estrutura familiar completa do mundo e cancro clinicamente confirmado, foi aproximadamente duas vezes maior na população dos 8 aos 20 anos, nascida de pais afetados ou irmãos de pessoas que não têm familiares. O risco de cancro do intestino delgado, testículo, tiroide e osso era cinco a oito vezes superior.
Assim sendo, espera-se que o aumento da incidência de cancro entre a população geriátrica seja um fator determinante para o crescimento do mercado global de testes de cancro hereditário.
Restrição
- Elevado custo dos testes de cancro hereditário
Os testes de cancro hereditário empregam produtos altamente avançados tecnologicamente. O desenvolvimento destes produtos envolve uma investigação e desenvolvimento rigorosos por parte do desenvolvedor. Desta forma, os custos dos procedimentos e dos produtos mantêm-se elevados, o que aumenta proporcionalmente o custo dos testes. Os kits de teste são caros porque exigem muitos recursos e envolvem médicos bem pagos, transporte e medicamentos dispendiosos.
- Além disso, os procedimentos de teste também têm sido utilizados em testes de cancro. No entanto, estes procedimentos são muito dispendiosos e podem estar associados a complicações e a piores resultados a longo prazo.
Assim, o elevado custo dos testes de cancro utilizando modalidades avançadas e produtos de tecnologia pode ser um importante fator de restrição para o crescimento do mercado global de testes de cancro hereditário.
Oportunidade
-
Iniciativas estratégicas dos participantes no mercado
O crescimento do mercado global de testes de cancro hereditário aumenta a necessidade de ideias estratégicas de negócio. Inclui uma parceria, expansão de negócios e outros desenvolvimentos. O aumento da procura de tratamento hereditário do cancro está a aumentar significativamente a procura de métodos de testes de diagnóstico. As estratégias planeadas permitem que os participantes do mercado se alinhem com as atividades funcionais da organização para atingir as metas definidas. Orienta as discussões e tomadas de decisão da empresa na determinação dos requisitos de recursos e orçamento para atingir os objetivos, aumentando assim a eficiência operacional.
Estas iniciativas estratégicas, tais como lançamentos de produtos, acordos e expansão de negócios pelos principais participantes do mercado, impulsionarão o crescimento do mercado e deverão funcionar como uma oportunidade para o mercado global de testes de cancro hereditário. Espera-se que as iniciativas estratégicas ajudem no crescimento e melhorem o portefólio de produtos da empresa, levando, em última análise, a mais geração de receitas. Assim, espera-se que estas iniciativas estratégicas dos participantes do mercado atuem como uma oportunidade de crescimento no mercado global de testes de cancro hereditário.
Desafio
- Desafios éticos enfrentados durante os testes hereditários de cancro
Durante os testes genéticos de cancro hereditário, um dos obstáculos éticos significativos é a falta de conhecimentos básicos dos profissionais de saúde sobre os testes genéticos e a sua falta de confiança na interpretação dos padrões de doenças familiares. O desafio para os profissionais de saúde é fornecer informação suficiente para fundamentar a tomada de decisão do doente e evidência para suportar o raciocínio por detrás de quaisquer sugestões que possam fazer.
A falta de reembolso cria barreiras económicas ao atendimento. O processo de avaliação de risco de cancro hereditário e aconselhamento é demorado, não sendo claro qual a melhor forma de documentar e cobrar por este serviço. Os oncologistas são muitas vezes forçados a navegar num ambiente de reembolso potencialmente incerto para testes genéticos, com diversas políticas de reembolso entre os pagadores externos.
Os testes genéticos para o cancro hereditário elevam questões éticas, que não podem ser resolvidas com os doentes ou com os seus familiares. Os vários aspetos de natureza ética, cultural e religiosa não devem ser uma barreira ao ato de testar o cancro hereditário. Todas estas são questões a resolver. Portanto, espera-se que os desafios éticos durante os testes de cancro hereditário desafiem o crescimento do mercado.
Impacto pós- COVID-19 no mercado global de testes de cancro hereditário
Muitos setores em todo o mundo foram prejudicados nos últimos 18 meses. Isto pode dever-se às grandes perturbações que os seus processos industriais e da cadeia de abastecimento estão a sofrer devido a várias medidas de precaução, tais como paragens e outras restrições que as instalações em todo o mundo têm vindo a seguir. O mesmo acontece com o mercado global de testes de cancro hereditário. Além disso, a procura de consumo diminuiu posteriormente, uma vez que as pessoas têm agora mais oportunidades de excluir as despesas não essenciais dos seus orçamentos, uma vez que as finanças gerais da maioria das pessoas foram severamente afectadas pelo boom. É possível esperar que estes fatores acima mencionados sobrecarreguem a margem de rendimento do mercado global de testes de cancro hereditário durante o período previsto.
Os fabricantes estão a tomar várias decisões estratégicas para recuperar após a COVID-19. Os participantes estão a conduzir diversas atividades de I&D, lançamento de produtos e parcerias estratégicas para melhorar a tecnologia e os resultados dos testes envolvidos no mercado de diagnóstico de transplante.
Desenvolvimentos recentes
- Em julho de 2022, a Helio Genomics e o seu parceiro de negócios, Fulgent Genetics (FLGT) anunciaram que a American Medical Association (AMA) emitiu um novo código de Análises Laboratoriais Proprietárias de Terminologia Processual Atual (CPT) de Categoria I para HelioLiver e adoção mais ampla de vigilância inovadora avançada testes para cancro do fígado nos EUA Isto ajudou a empresa a expandir o seu portfólio de produtos.
- Em março de 2022, a Illumina, Inc. lançou o kit de diagnóstico in vitro (IVD), um sequenciador de RNA do cancro. O lançamento resultou na expansão da linha de produtos de sequenciação, seguida da aprovação pós-comercialização.
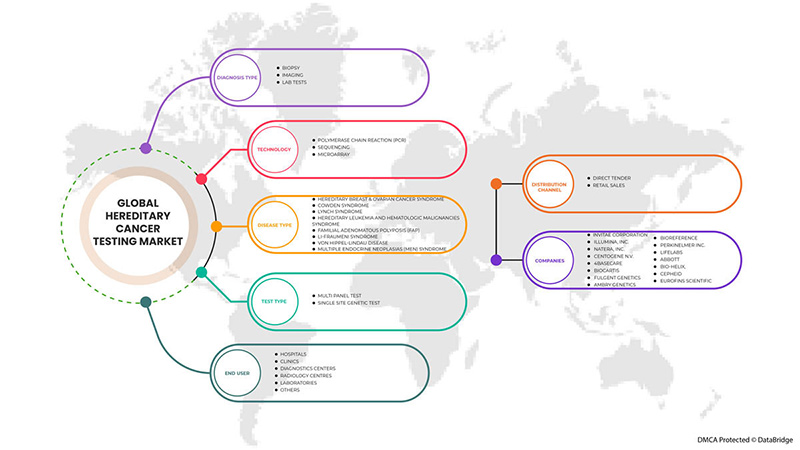
Âmbito do mercado global de testes de cancro hereditário
O mercado global de testes de cancro hereditário está segmentado em tipo de teste, tipo de diagnóstico, tecnologia, tipo de doença, utilizador final e canal de distribuição. O crescimento entre segmentos ajuda-o a analisar os nichos de crescimento e as estratégias para abordar o mercado e determinar as suas principais áreas de aplicação e a diferença nos seus mercados-alvo.
POR TIPO DE TESTE
- TESTE DE PAINEL MÚLTIPLO
- TESTE GENÉTICO DE SÍTIO ÚNICO
Com base no tipo de teste, o mercado global de testes de cancro hereditário está segmentado em testes de múltiplos painéis e testes genéticos de local único.
POR TIPO DE DIAGNÓSTICO
- BIÓPSIA
- IMAGEM
- TESTES DE LABORATÓRIO
Com base no tipo de diagnóstico, o mercado global de testes de cancro hereditário está segmentado em biópsia, imagiologia e exames laboratoriais.
POR TECNOLOGIA
- SEQUENCIAMENTO
- REAÇÃO EM CADEIA DA POLIMERASE (PCR)
- MICROARRAY
Com base na tecnologia, o mercado global de testes de cancro hereditário está segmentado em sequenciação, reação em cadeia da polimerase (PCR) e microarray.
POR TIPO DE DOENÇA
- SÍNDROME HEREDITÁRIA DO CANCRO DA MAMA E DO OVÁRIO
- SÍNDROME DE COWDEN
- SÍNDROME DE LYNCH
- LEUCEMIA HEREDITÁRIA E SÍNDROMES DE MALIGNAS HEMATOLÓGICAS
- POLIPOSE ADENOMATOSA FAMILIAR (PAF)
- SÍNDROME DE LI-FRAUMENI
- DOENÇA DE VON HIPPEL-LINDAU
- SÍNDROMES DE NEOPLASIAS ENDÓCRINAS MÚLTIPLAS (HOMENS)
Com base no tipo de doença, o mercado global de testes de cancro hereditário está segmentado em síndrome de cancro hereditário da mama e do ovário, síndrome de Cowden, síndrome de Lynch, leucemia hereditária e síndromes de malignidades hematológicas, polipose adenomatosa familiar (FAP), síndrome de Li-Fraumeni, vol- doença de hippel lindau, síndrome de neoplasias endócrinas múltiplas (NEM).
POR UTILIZADOR FINAL
- HOSPITAIS
- CLÍNICAS
- LABORATÓRIOS
- CENTROS DE RADIOLOGIA
- CENTROS DE DIAGNÓSTICO
- OUTROS
Com base no utilizador final, o mercado global de testes de cancro hereditário está segmentado em hospitais, clínicas, laboratórios, centros de radiologia, centros de diagnóstico e outros.
POR CANAL DE DISTRIBUIÇÃO
- LICITAÇÃO DIRETA
- VENDAS NO VAREJO
Com base no canal de distribuição, o mercado global de testes de cancro hereditário está segmentado em licitação direta e vendas a retalho.
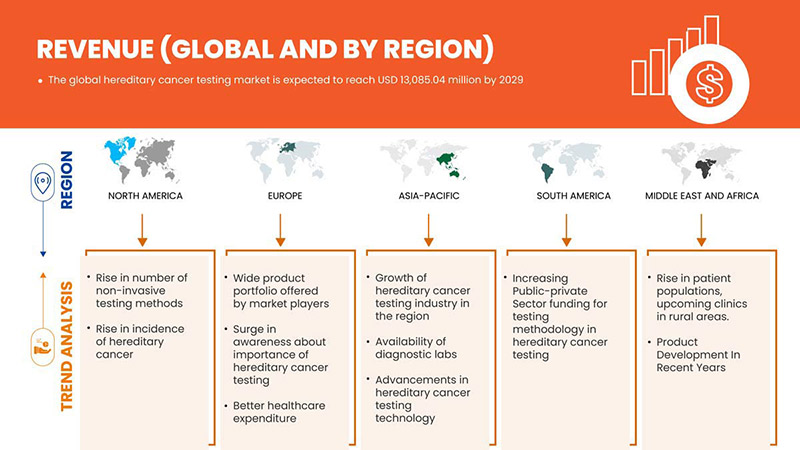
Análise/Insights Regionais do Mercado Global de Testes de Cancro Hereditário
O mercado global de testes de cancro hereditário é analisado e são fornecidas informações sobre o tamanho do mercado por país, tipo de teste, tipo de diagnóstico, tecnologia, tipo de doença, utilizador final e canal de distribuição.
Os países abrangidos neste relatório de mercado: EUA, Canadá, México, Alemanha, França, Reino Unido, Itália, Espanha, Rússia, Turquia, Bélgica, Países Baixos, Suíça e restante Europa, China, Japão, Índia, Coreia do Sul, Singapura, Tailândia, Malásia, Austrália, Filipinas, Indonésia e restante Ásia-Pacífico, África do Sul, Arábia Saudita, Emirados Árabes Unidos, Egito, Israel e restante Médio Oriente e África, Brasil, Argentina e restante América do Sul .
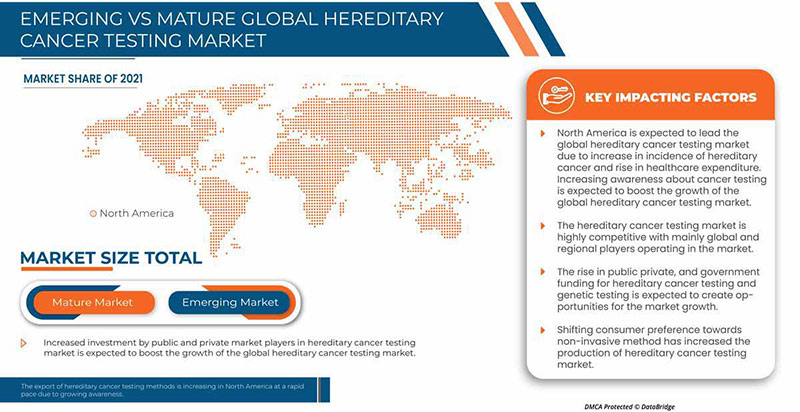
A América do Norte está a dominar o mercado devido ao crescente investimento em I&D, o que deverá impulsionar o crescimento do mercado. Os EUA dominam a região da América do Norte devido à forte presença dos principais participantes, Invitae Corporation, Illumina, Inc., Natera, Inc. e outros. O Reino Unido domina a região da Europa devido à produção em massa de testes hereditários de cancro e à crescente procura dos mercados emergentes e à expansão dos sectores da saúde. A China domina a região Ásia-Pacífico devido ao aumento dos testes de diagnóstico relacionados com o cancro.
A secção do relatório sobre os países também fornece fatores individuais que impactam o mercado e alterações na regulamentação do mercado nacional que impactam as tendências atuais e futuras do mercado. Pontos de dados como novas vendas, vendas de reposição, demografia do país, atos regulamentares e tarifas de importação e exportação são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e a disponibilidade de marcas globais e os desafios enfrentados devido à grande ou escassa concorrência de marcas locais e nacionais, e o impacto dos canais de vendas são considerados ao fornecer uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado global de testes de cancro hereditário
O panorama competitivo do mercado global de testes de cancro hereditário fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em I&D, novas iniciativas de mercado, localizações e instalações de produção, pontos fortes e fracos da empresa, lançamento de produtos, pipelines de testes de produto, aprovações de produto, patentes, amplitude e extensão do produto domínio da aplicação, curva de vida da tecnologia. Os pontos de dados fornecidos acima estão apenas relacionados com o foco da empresa no mercado global de testes de cancro hereditário.
Alguns dos principais participantes que operam no mercado global de testes de cancro hereditário são a Invitae Corporation, Illumina, Inc., Natera, Inc., CENTOGENE NV, 4baseCare, Biocartis, Fulgent Genetics, Ambry Genetics, BioReference, PerkinElmer Inc., LifeLabs, Abbott , BIO-HELIX, Cepheid, Eurofins Scientific, entre outros.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 GLOBAL HEREDITARY CANCER TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF HEREDITARY CANCER
6.1.2 INCREASE IN THE GERIATRIC POPULATION
6.1.3 RISING HEALTHCARE SPENDING
6.1.4 INCREASE IN AWARENESS ABOUT CANCER TESTING
6.2 RESTRAINTS
6.2.1 HIGH COST OF HEREDITARY CANCER TESTING
6.2.2 LACK OF SKILLED PROFESSIONALS
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.2 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
6.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE AND EARLY DIAGNOSIS
6.3.4 TECHNOLOGICAL DEVELOPMENTS IN HEREDITARY CANCER TESTING PROCEDURES
6.4 CHALLENGES
6.4.1 ETHICAL CHALLENGES FACED DURING HEREDITARY CANCER TESTING
6.4.2 RISING COMPETITION AMONG MARKET PLAYERS
7 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 MULTI PANEL TEST
7.3 SINGLE-SITE GENETIC TEST
8 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE
8.1 OVERVIEW
8.2 BIOPSY
8.2.1 NEEDLE BIOPSIES
8.2.2 ENDOSCOPIC BIOPSIES
8.2.3 LAPAROSCOPIC, THORACOSCOPIC, AND MEDIASTINOSCOPIC BIOPSY
8.2.4 LAPAROTOMY AND THORACOTOMY
8.2.5 OTHERS
8.3 IMAGING
8.3.1 MAGNETIC RESONANCE IMAGING (MRI)
8.3.2 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 NUCLEAR SCAN
8.3.5 ULTRASOUND
8.3.6 X-RAYS
8.4 LAB TESTS
8.4.1 BLOOD
8.4.2 URINE
8.4.3 OTHERS
9 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 POLYMERASE CHAIN REACTION (PCR)
9.3 SEQUENCING
9.4 MICRO ARRAY
10 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 HEREDITARY BREAST & OVARIAN CANCER SYNDROME
10.3 COWDEN SYNDROME
10.4 LYNCH SYNDROME
10.5 HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME
10.6 FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
10.7 LI-FRAUMENI SYNDROME
10.8 VON HIPPEL-LINDAU DISEASE
10.9 MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME
11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 DIAGNOSTIC CENTERS
11.5 RADIOLOGY CENTERS
11.6 LABORATORIES
11.7 OTHERS
12 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
13 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION
13.1 OVERVIEW
13.2 ASIA-PACIFIC
13.2.1 CHINA
13.2.2 JAPAN
13.2.3 SOUTH KOREA
13.2.4 INDIA
13.2.5 AUSTRALIA
13.2.6 SINGAPORE
13.2.7 THAILAND
13.2.8 MALAYSIA
13.2.9 INDONESIA
13.2.10 PHILIPPINES
13.2.11 REST OF ASIA-PACIFIC
13.3 NORTH AMERICA
13.3.1 U.S.
13.3.2 CANADA
13.3.3 MEXICO
13.4 EUROPE
13.4.1 GERMANY
13.4.2 FRANCE
13.4.3 U.K.
13.4.4 RUSSIA
13.4.5 ITALY
13.4.6 SPAIN
13.4.7 TURKEY
13.4.8 NETHERLANDS
13.4.9 SWITZERLAND
13.4.10 BELGIUM
13.4.11 REST OF EUROPE
13.5 SOUTH AMERICA
13.5.1 BRAZIL
13.5.2 ARGENTINA
13.5.3 REST OF SOUTH AMERICA
13.6 MIDDLE EAST AND AFRICA
13.6.1 SOUTH AFRICA
13.6.2 SAUDI ARABIA
13.6.3 U.A.E.
13.6.4 EGYPT
13.6.5 ISRAEL
13.6.6 REST OF THE MIDDLE EAST AND AFRICA
14 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ABBOTT
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 ILLUMINA, INC. (2021)
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PERKINELMER INC. (2021)
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 LIFELABS GENETICS
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 EUROFINS SCIENTIFIC (2021)
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 AMBRY GENETICS
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 BIOCARTIS
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 BIO-HELIX
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOREFERENCE (A SUBSIDIARY OF OPKO HEALTH, INC.) (2021)
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CENTOGENE N.V. (2021)
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 FULGENT GENETICS
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 INVITAE CORPORATION
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 NATERA, INC. (2021)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 4BASECARE.
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Lista de Tabela
TABLE 1 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 2 GLOBAL MULTI PANEL TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 GLOBAL SINGLE-SITE GENETIC TEST IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 5 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 GLOBAL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 7 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 GLOBAL IMAGING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 9 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 GLOBAL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 11 GLOBAL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 12 GLOBAL POLYMERASE CHAIN REACTION (PCR) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 GLOBAL SEQUENCING IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 GLOBAL MICROARRAY IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 16 GLOBAL HEREDITARY BREAST & OVARIAN CANCER SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 GLOBAL COWDEN SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 GLOBAL LYNCH SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 GLOBAL HEREDITARY LEUKEMIA AND HEMATOLOGIC MALIGNANCIES SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 GLOBAL FAMILIAL ADENOMATOUS POLYPOSIS (FAP) IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 GLOBAL LI-FRAUMENI SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 GLOBAL VON HIPPEL-LINDAU DISEASE IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 GLOBAL MULTIPLE ENDOCRINE NEOPLASIAS (MEN) SYNDROME IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 GLOBAL HEREDITARY CANCER TESTING MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 25 GLOBAL HOSPITALS CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 GLOBAL CLINICS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 GLOBAL DIAGNOSTIC CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 GLOBAL RADIOLOGY CENTERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 GLOBAL LABORATORIES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 GLOBAL OTHERS IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 GLOBAL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 GLOBAL DIRECT TENDER IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 GLOBAL RETAIL SALES IN HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 GLOBAL HEREDITARY CANCER TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 43 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 44 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 46 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 47 CHINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 48 CHINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 50 CHINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 51 CHINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 52 CHINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 53 CHINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 54 CHINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 55 CHINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 57 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 58 JAPAN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 59 JAPAN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 60 JAPAN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 61 JAPAN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 62 JAPAN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 63 JAPAN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 64 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 65 JAPAN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 66 JAPAN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 67 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 69 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 70 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 71 SOUTH KOREA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 72 SOUTH KOREA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 73 SOUTH KOREA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 74 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 75 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 77 SOUTH KOREA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 79 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 80 INDIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 81 INDIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 82 INDIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 83 INDIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 84 INDIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 85 INDIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 86 INDIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 87 INDIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 88 INDIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 89 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 91 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 92 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 93 AUSTRALIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 94 AUSTRALIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 95 AUSTRALIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 96 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 97 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 98 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 99 AUSTRALIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 100 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 101 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 102 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 103 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 104 SINGAPORE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 105 SINGAPORE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 106 SINGAPORE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 107 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 108 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 109 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 110 SINGAPORE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 111 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 113 THAILAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 114 THAILAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 115 THAILAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 116 THAILAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 117 THAILAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 118 THAILAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 119 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 120 THAILAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 121 THAILAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 122 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 123 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 124 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 125 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 126 MALAYSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 127 MALAYSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 128 MALAYSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 129 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 130 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 131 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 132 MALAYSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 133 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 135 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 136 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 137 INDONESIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 138 INDONESIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 139 INDONESIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 140 INDONESIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 141 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 142 INDONESIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 143 INDONESIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 144 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 145 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 146 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 147 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 148 PHILIPPINES BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 149 PHILIPPINES IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 150 PHILIPPINES LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 151 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 152 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 153 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 154 PHILIPPINES HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 155 REST OF ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 157 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 158 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 159 NORTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 160 NORTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 161 NORTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 162 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 163 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 164 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 165 NORTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 166 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 167 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 168 U.S. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 169 U.S. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 170 U.S. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 171 U.S. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 172 U.S. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 173 U.S. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 174 U.S. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 175 U.S. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 176 U.S. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 177 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 179 CANADA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 180 CANADA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 181 CANADA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 182 CANADA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 183 CANADA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 184 CANADA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 185 CANADA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 186 CANADA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 187 CANADA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 188 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 189 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 190 MEXICO HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 191 MEXICO HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 192 MEXICO BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 193 MEXICO IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 194 MEXICO LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 195 MEXICO HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 196 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 197 MEXICO HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 198 MEXICO HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 199 EUROPE HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 200 EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 201 EUROPE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 202 EUROPE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 203 EUROPE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 204 EUROPE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 205 EUROPE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 206 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 207 EUROPE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 208 EUROPE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 209 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 210 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 211 GERMANY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 212 GERMANY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 213 GERMANY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 214 GERMANY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 215 GERMANY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 216 GERMANY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 217 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 218 GERMANY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 219 GERMANY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 220 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 221 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 222 FRANCE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 223 FRANCE HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 224 FRANCE BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 225 FRANCE IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 226 FRANCE LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 227 FRANCE HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 228 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 229 FRANCE HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 230 FRANCE HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 231 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 232 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 233 U.K. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 234 U.K. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 235 U.K. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 236 U.K. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 237 U.K. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 238 U.K. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 239 U.K. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 240 U.K. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 241 U.K. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 243 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 244 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 245 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 246 RUSSIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 247 RUSSIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 248 RUSSIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 249 RUSSIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 250 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 251 RUSSIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 252 RUSSIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 253 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 254 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 255 ITALY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 256 ITALY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 257 ITALY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 258 ITALY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 259 ITALY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 260 ITALY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 261 ITALY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 262 ITALY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 263 ITALY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 264 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 265 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 266 SPAIN HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 267 SPAIN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 268 SPAIN BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 269 SPAIN IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 270 SPAIN LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 271 SPAIN HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 272 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 273 SPAIN HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 274 SPAIN HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 275 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 276 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 277 TURKEY HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 278 TURKEY HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 279 TURKEY BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 280 TURKEY IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 281 TURKEY LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 282 TURKEY HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 283 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 284 TURKEY HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 285 TURKEY HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 286 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 287 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 288 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 289 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 290 NETHERLANDS BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 291 NETHERLANDS IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 292 NETHERLANDS LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 293 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 294 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 295 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 296 NETHERLANDS HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 297 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 298 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 299 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 300 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 301 SWITZERLAND BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 302 SWITZERLAND IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 303 SWITZERLAND LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 304 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 305 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 306 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 307 SWITZERLAND HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 310 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 311 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 312 BELGIUM BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 313 BELGIUM IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 314 BELGIUM LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 315 BELGIUM HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 316 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 317 BELGIUM HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 318 BELGIUM HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 319 REST OF EUROPE HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 320 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 321 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 322 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 323 SOUTH AMERICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 324 SOUTH AMERICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 325 SOUTH AMERICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 326 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 327 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 328 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 329 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 330 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 331 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 332 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 333 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 334 BRAZIL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 335 BRAZIL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 336 BRAZIL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 337 BRAZIL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 338 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 339 BRAZIL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 340 BRAZIL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 341 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 342 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 343 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 344 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 345 ARGENTINA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 346 ARGENTINA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 347 ARGENTINA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 348 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 349 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 350 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 351 ARGENTINA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 352 REST OF THE SOUTH AMERICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 353 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 354 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 355 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 356 MIDDLE EAST AND AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 357 MIDDLE EAST AND AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 358 MIDDLE EAST AND AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 359 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 360 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 361 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 362 MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 363 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 364 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 365 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 366 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 367 SOUTH AFRICA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 368 SOUTH AFRICA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 369 SOUTH AFRICA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 370 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 371 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 372 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 373 SOUTH AFRICA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 374 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 375 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 376 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 377 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 378 SAUDI ARABIA BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 379 SAUDI ARABIA IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 380 SAUDI ARABIA LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 381 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 382 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 383 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 384 SAUDI ARABIA HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 385 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 386 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 387 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 388 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 389 U.A.E. BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 390 U.A.E. IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 391 U.A.E. LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 392 U.A.E. HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 393 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 394 U.A.E. HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 395 U.A.E. HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 396 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 397 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 398 EGYPT HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 399 EGYPT HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 400 EGYPT BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 401 EGYPT IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 402 EGYPT LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 403 EGYPT HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 404 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 405 EGYPT HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 406 EGYPT HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 407 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 408 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (UNITS)
TABLE 409 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 ASP (USD)
TABLE 410 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 411 ISRAEL BIOPSY IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 412 ISRAEL IMAGINING IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 413 ISRAEL LAB TESTS IN HEREDITARY CANCER TESTING MARKET, BY DIAGNOSIS TYPE, 2020-2029 (USD MILLION)
TABLE 414 ISRAEL HEREDITARY CANCER TESTING MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 415 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISEASE TYPE, 2020-2029 (USD MILLION)
TABLE 416 ISRAEL HEREDITARY CANCER TESTING MARKET, BY END USERS, 2020-2029 (USD MILLION)
TABLE 417 ISRAEL HEREDITARY CANCER TESTING MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 418 REST OF MIDDLE EAST AND AFRICA HEREDITARY CANCER TESTING MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
Lista de Figura
FIGURE 1 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 2 GLOBAL HEREDITARY CANCER TESTING MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL HEREDITARY CANCER TESTING MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL HEREDITARY CANCER TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL HEREDITARY CANCER TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL HEREDITARY CANCER TESTING MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 GLOBAL HEREDITARY CANCER TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL HEREDITARY CANCER TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL HEREDITARY CANCER TESTING MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE GLOBAL HEREDITARY CANCER TESTING MARKET, AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 12 EXPANDING REPRODUCTIVE GENETIC HEALTH SPACE IS EXPECTED TO DRIVE THE GLOBAL HEREDITARY CANCER TESTING MARKET IN THE FORECAST PERIOD
FIGURE 13 MULTI PANEL TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL HEREDITARY CANCER TESTING MARKET IN 2022 & 2029
FIGURE 14 NORTH AMERICA IS THE FASTEST-GROWING MARKET FOR HEREDITARY CANCER TESTING MANUFACTURERS IN THE FORECAST PERIOD
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL HEREDITARY CANCER TESTING MARKET
FIGURE 16 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2021
FIGURE 17 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 18 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 19 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2021
FIGURE 21 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, 2022-2029 (USD MILLION)
FIGURE 22 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, CAGR (2022-2029)
FIGURE 23 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DIAGNOSIS TYPE, LIFELINE CURVE
FIGURE 24 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2021
FIGURE 25 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, 2020-2029 (USD MILLION)
FIGURE 26 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 27 GLOBAL HEREDITARY CANCER TESTING MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 28 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2021
FIGURE 29 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, 2022-2029 (USD MILLION)
FIGURE 30 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, CAGR (2022-2029)
FIGURE 31 GLOBAL HEREDITARY CANCER TESTING MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 32 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2021
FIGURE 33 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, 2020-2029 (USD MILLION)
FIGURE 34 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, CAGR (2022-2029)
FIGURE 35 GLOBAL HEREDITARY CANCER TESTING MARKET : BY END USER, LIFELINE CURVE
FIGURE 36 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2021
FIGURE 37 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 38 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 39 GLOBAL HEREDITARY CANCER TESTING MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 GLOBAL HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 41 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021)
FIGURE 42 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2022 & 2029)
FIGURE 43 GLOBAL HEREDITARY CANCER TESTING MARKET: BY REGION (2021 & 2029)
FIGURE 44 GLOBAL HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 45 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 46 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 47 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 48 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 49 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 50 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 51 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 52 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 53 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 54 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 55 EUROPE HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 60 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 61 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 62 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 63 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 64 SOUTH AMERICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 65 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: SNAPSHOT (2021)
FIGURE 66 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021)
FIGURE 67 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 68 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 69 MIDDLE EAST & AFRICA HEREDITARY CANCER TESTING MARKET: BY TEST TYPE (2022-2029)
FIGURE 70 GLOBAL HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 71 NORTH AMERICA HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 72 EUROPE HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)
FIGURE 73 ASIA-PACIFIC HEREDITARY CANCER TESTING MARKET: COMPANY SHARE 2021 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.