Global Clinical Trial Packaging And Labelling Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
854.43 Billion
USD
1,468.07 Billion
2025
2033
USD
854.43 Billion
USD
1,468.07 Billion
2025
2033
| 2026 –2033 | |
| USD 854.43 Billion | |
| USD 1,468.07 Billion | |
|
|
|
|
Segmentação do mercado global de embalagens e rotulagem para ensaios clínicos, por tipo de medicamento (medicamentos de pequenas moléculas e medicamentos biológicos), fase (fase I, fase II, fase III, fase IV e estudos de bioequivalência/biodisponibilidade), área terapêutica (oncologia, distúrbios neurológicos e mentais, doenças infecciosas e do sistema imunológico, doenças do sistema digestivo, distúrbios sanguíneos e outras áreas terapêuticas) - Tendências e previsões do setor até 2033.
Tamanho do mercado de embalagens e rotulagem para ensaios clínicos
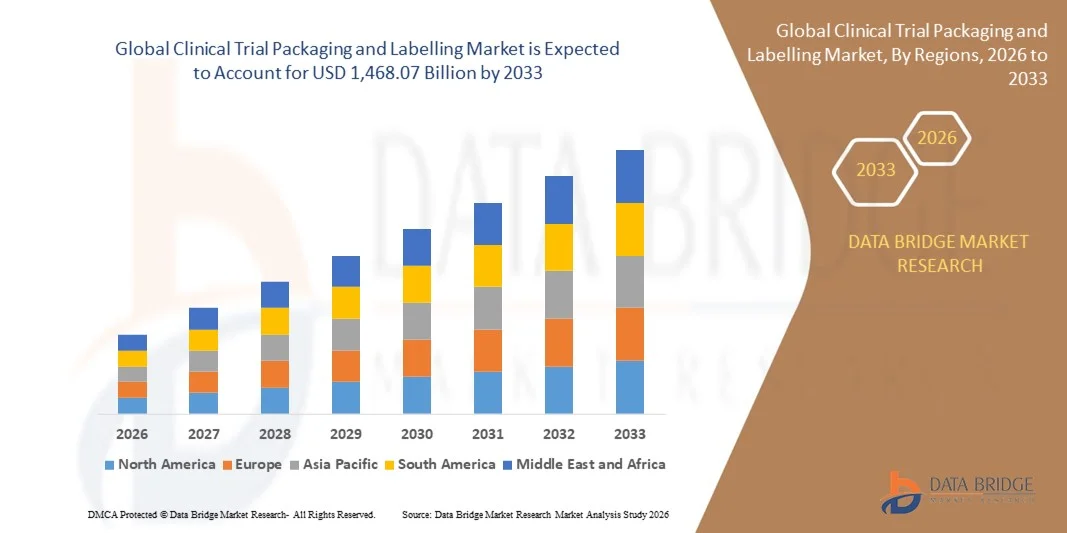
- O mercado global de embalagens e rotulagem para ensaios clínicos foi avaliado em US$ 854,43 bilhões em 2025 e deverá atingir US$ 1.468,07 bilhões até 2033 , com uma taxa de crescimento anual composta (CAGR) de 7,00% durante o período de previsão.
- O crescimento do mercado é impulsionado principalmente pela crescente demanda por soluções de embalagens seguras, em conformidade com as normas e centradas no paciente para ensaios clínicos, garantindo a integridade do produto e a adesão aos padrões regulatórios.
- A crescente complexidade dos ensaios clínicos, incluindo os de produtos biológicos e medicamentos personalizados, está impulsionando a necessidade de soluções inovadoras de embalagem e rotulagem que melhorem a eficiência dos ensaios e a adesão do paciente.
Análise de mercado de embalagens e rotulagem para ensaios clínicos
- O mercado está testemunhando inovações significativas em materiais de embalagem sustentáveis e invioláveis, aprimorando a conformidade ambiental e os padrões de segurança.
- A crescente terceirização de serviços de ensaios clínicos por empresas farmacêuticas e de biotecnologia está impulsionando a demanda por fornecedores especializados em embalagens e rotulagem com recursos avançados.
- A América do Norte dominou o mercado de embalagens e rotulagem para ensaios clínicos, com a maior participação na receita em 2025, impulsionada pelo crescente número de ensaios clínicos, infraestrutura de saúde avançada e adoção cada vez maior de soluções de embalagem centradas no paciente.
- A região Ásia-Pacífico deverá apresentar a maior taxa de crescimento no mercado global de embalagens e rotulagem para ensaios clínicos , impulsionada pela expansão das atividades de ensaios clínicos, iniciativas governamentais favoráveis e soluções de fabricação e embalagem com boa relação custo-benefício.
- O segmento de medicamentos biológicos detinha a maior participação na receita de mercado em 2025, impulsionado pelo crescente desenvolvimento de vacinas, anticorpos monoclonais e terapias celulares e gênicas que exigem embalagens especializadas com controle de temperatura, invioláveis e centradas no paciente. As embalagens de produtos biológicos frequentemente integram tecnologias de monitoramento digital e rastreamento para garantir a estabilidade do produto e a conformidade regulatória em ensaios clínicos multicêntricos.
Escopo do relatório e segmentação do mercado de embalagens e rotulagem para ensaios clínicos
|
Atributos |
Principais informações de mercado sobre embalagens e rotulagem para ensaios clínicos |
|
Segmentos abrangidos |
|
|
Países abrangidos |
América do Norte
Europa
Ásia-Pacífico
Oriente Médio e África
Ámérica do Sul
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além das informações sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado elaborados pela Data Bridge Market Research também incluem análise de importação e exportação, visão geral da capacidade de produção, análise de produção e consumo, análise de tendências de preços, cenário de mudanças climáticas, análise da cadeia de suprimentos, análise da cadeia de valor, visão geral de matérias-primas/insumos, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de embalagens e rotulagem para ensaios clínicos
Adoção de soluções de embalagem avançadas e centradas no paciente
- A crescente ênfase em embalagens avançadas e centradas no paciente para ensaios clínicos está transformando as indústrias farmacêutica e biotecnológica, aprimorando a segurança, a rastreabilidade e a adesão aos medicamentos. Soluções de embalagem personalizadas ajudam a garantir a dosagem precisa e a reduzir erros de medicação, melhorando os resultados dos ensaios e a adesão do paciente. Além disso, essas soluções auxiliam nas submissões regulatórias, mantendo a documentação completa e aumentando a responsabilidade em ensaios multicêntricos.
- A crescente demanda por sistemas de embalagens inteligentes, invioláveis e com controle de temperatura está acelerando sua adoção tanto em ensaios clínicos de fase inicial quanto de fase tardia. Essas soluções permitem o monitoramento em tempo real de produtos experimentais sensíveis, principalmente biológicos e vacinas, garantindo a integridade em toda a cadeia de suprimentos. Além disso, a adoção da logística da cadeia de frio e de sensores inteligentes permite que os patrocinadores detectem desvios precocemente e reduzam o risco de deterioração do produto.
- A integração de tecnologias de etiquetagem digital, incluindo RFID, códigos QR e sistemas de rastreamento, está aprimorando a transparência da cadeia de suprimentos e a conformidade regulatória. Patrocinadores e CROs se beneficiam de recursos de monitoramento aprimorados, preparação para auditorias e risco minimizado de recalls de produtos. Além disso, essas tecnologias facilitam o gerenciamento eficiente de estoque, reduzem erros manuais e simplificam a geração de relatórios para ensaios clínicos globais.
- Por exemplo, em 2023, diversas empresas de embalagem terceirizada da América do Norte e da Europa implementaram soluções de rotulagem inteligente para ensaios clínicos multicêntricos, resultando em maior integridade de dados, segurança do paciente e conformidade regulatória. Essas implementações também levaram a um processamento de remessas mais rápido, melhor rastreamento de produtos em investigação e maior conformidade com as diretrizes do ICH e do FDA.
- Embora a adoção de embalagens e rotulagem avançadas esteja crescendo rapidamente, o crescimento sustentado depende da inovação, da padronização de soluções digitais e de estratégias de implementação com boa relação custo-benefício em diversos cenários de ensaios clínicos. Os patrocinadores estão cada vez mais focados em soluções escaláveis que possam se adaptar a diferentes tamanhos de ensaios, categorias terapêuticas e localizações geográficas para maximizar a eficiência.
Dinâmica do mercado de embalagens e rotulagem para ensaios clínicos
Motorista
Crescente demanda por embalagens seguras, em conformidade com as normas e eficientes para ensaios clínicos.
- O aumento das atividades globais de ensaios clínicos e a complexidade dos protocolos de estudo estão impulsionando a necessidade de soluções de embalagem e rotulagem seguras, em conformidade com as normas e eficientes. Os patrocinadores buscam sistemas confiáveis para manter a qualidade dos medicamentos, prevenir a falsificação e garantir a conformidade regulatória em todas as regiões. Além disso, a crescente ênfase na segurança e adesão do paciente em ensaios descentralizados está impulsionando ainda mais a demanda por embalagens seguras e fáceis de usar.
- A crescente adoção de produtos biológicos, medicamentos personalizados e terapias experimentais exige soluções de embalagem avançadas que ofereçam controle de temperatura, proteção contra luz e umidade, além de inviolabilidade. Essas exigências impulsionam a inovação e os investimentos em formatos de embalagem especializados. A integração com monitoramento digital e soluções baseadas em IoT também permite o rastreamento em tempo real da condição do produto e ações corretivas mais rápidas durante o transporte.
- A expansão das organizações de pesquisa e embalagem por contrato, juntamente com parcerias entre empresas farmacêuticas e fornecedores de tecnologia, está facilitando o acesso a soluções de embalagem escaláveis e de alta qualidade. Isso permite o início mais rápido dos ensaios clínicos e uma melhor gestão do produto em múltiplos locais. Os esforços colaborativos também estão promovendo o compartilhamento de conhecimento, a transferência de tecnologia e a otimização de custos nas cadeias de suprimentos de ensaios clínicos.
- Por exemplo, em 2023, o mercado americano testemunhou uma forte adoção de seringas pré-carregadas e soluções de embalagem blister em ensaios clínicos de Fase II e III, impulsionada pela necessidade de segurança do paciente e eficiência dos ensaios. A integração de serialização, lacres invioláveis e rotulagem inteligente aprimorou ainda mais a adesão e reduziu erros logísticos em ensaios multicêntricos complexos.
- Embora a demanda seja forte, a inovação contínua, a automação e a integração de soluções digitais permanecem essenciais para dar suporte a protocolos complexos de ensaios clínicos e à conformidade com as regulamentações globais. O mercado também está explorando cada vez mais designs de embalagens modulares e flexíveis que possam acomodar uma ampla gama de formatos de produtos e condições de envio.
Restrição/Desafio
Alto custo de soluções de embalagens especializadas e complexidade regulatória.
- O alto custo das embalagens avançadas para ensaios clínicos, incluindo soluções com controle de temperatura, inteligentes e invioláveis, limita a adoção por pequenas empresas de biotecnologia e patrocinadores de projetos em estágio inicial. Restrições orçamentárias frequentemente exigem a priorização das atividades essenciais do ensaio em detrimento de investimentos em embalagens sofisticadas. Além disso, os altos requisitos de capital inicial para equipamentos automatizados e sistemas de rotulagem digital representam barreiras de entrada em mercados emergentes.
- A complexidade regulatória em diferentes regiões, incluindo requisitos de rotulagem, mandatos de serialização e registro eletrônico de dados, aumenta os custos de conformidade e os desafios operacionais. Os patrocinadores precisam navegar pelos padrões específicos de cada país para evitar atrasos e penalidades. As atualizações contínuas das diretrizes por agências como FDA, EMA e PMDA também exigem treinamento constante e atualizações de sistemas, aumentando ainda mais a pressão financeira e operacional.
- O conhecimento ou a experiência limitados com soluções de rotulagem digital e embalagem automatizada em mercados emergentes restringem a adoção dessas tecnologias. Os patrocinadores de ensaios clínicos podem enfrentar desafios logísticos em regiões remotas ou subdesenvolvidas, afetando a integridade do produto e a segurança do paciente. Além disso, a infraestrutura local insuficiente para o gerenciamento da cadeia de frio e o transporte seguro pode dificultar ainda mais a implementação generalizada de tecnologias avançadas de embalagem.
- Por exemplo, em 2023, vários mercados do Sudeste Asiático relataram uma adoção mais lenta de tecnologias de embalagens inteligentes devido aos altos custos e incertezas regulatórias, apesar do crescente número de ensaios clínicos. Os patrocinadores frequentemente optaram por métodos de embalagem convencionais para minimizar despesas, o que impactou a visibilidade do produto, a conformidade e os recursos centrados no paciente.
- Embora as tecnologias e os materiais continuem a evoluir, abordar os desafios relacionados a custos, conformidade regulatória e adoção regional é fundamental para o crescimento a longo prazo do mercado global de embalagens e rotulagem para ensaios clínicos. As partes interessadas estão investindo cada vez mais em treinamento, parcerias e soluções escaláveis para superar essas barreiras e otimizar a eficiência dos ensaios em todo o mundo.
Escopo do mercado de embalagens e rotulagem para ensaios clínicos
O mercado de embalagens e rotulagem para ensaios clínicos é segmentado com base no tipo de medicamento, fase e área terapêutica.
- Por tipo de medicamento
Com base no tipo de medicamento, o mercado é segmentado em medicamentos de pequenas moléculas e medicamentos biológicos. O segmento de medicamentos biológicos detinha a maior participação na receita de mercado em 2025, impulsionado pelo crescente desenvolvimento de vacinas, anticorpos monoclonais e terapias celulares e gênicas que exigem embalagens especializadas com controle de temperatura, invioláveis e centradas no paciente. As embalagens de produtos biológicos frequentemente integram tecnologias de monitoramento digital e rastreamento para garantir a estabilidade do produto e a conformidade regulatória em ensaios clínicos multicêntricos.
O segmento de medicamentos de pequenas moléculas deverá apresentar a taxa de crescimento mais rápida entre 2026 e 2033, impulsionado pelo número crescente de formulações orais sólidas e líquidas em desenvolvimento clínico e pela adoção de soluções de embalagem convenientes, como blisters e formatos de dose unitária. Essas soluções melhoram a precisão da dosagem, a adesão do paciente ao tratamento e reduzem o risco de contaminação, tornando-as cada vez mais preferidas em estudos de fases iniciais e avançadas.
- Por fase
Com base na fase, o mercado é segmentado em estudos de Fase I, Fase II, Fase III, Fase IV e bioequivalência/biodisponibilidade (BA/BE). O segmento de Fase III detinha a maior participação na receita em 2025, devido aos protocolos complexos dos estudos, às grandes populações de pacientes e aos rigorosos requisitos regulatórios que exigem soluções avançadas de embalagem para garantir a segurança, a rastreabilidade e a conformidade do produto. As embalagens para Fase III geralmente envolvem distribuição em múltiplos locais e exigem serialização robusta e recursos invioláveis.
Espera-se que o segmento de Fase I apresente a taxa de crescimento mais rápida de 2026 a 2033, impulsionado pelo número crescente de ensaios clínicos em estágio inicial e pela demanda cada vez maior por soluções de embalagem centradas no paciente, flexíveis e escaláveis. Essas soluções ajudam a manter a integridade do produto, otimizar a logística dos ensaios e aumentar a adesão dos participantes em estudos de fase I.
- Por área terapêutica
Com base na área terapêutica, o mercado é segmentado em oncologia, distúrbios neurológicos e mentais, doenças infecciosas e do sistema imunológico, doenças do sistema digestivo, distúrbios sanguíneos e outras áreas terapêuticas. O segmento de oncologia detinha a maior participação na receita de mercado em 2025, impulsionado pelo crescente número de ensaios clínicos de câncer, regimes de dosagem complexos e medicamentos experimentais de alto valor que exigem embalagens especializadas para segurança, controle de temperatura e conformidade regulatória.
O segmento de doenças infecciosas e do sistema imunológico deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado pelo aumento dos investimentos em pesquisa e desenvolvimento, pelo desenvolvimento de vacinas e por terapias biológicas que exigem soluções de cadeia de frio e embalagens invioláveis. A adoção de rotulagem digital e sistemas de monitoramento inteligente aprimora ainda mais a segurança do paciente e a eficiência dos ensaios clínicos nessa área terapêutica.
Análise Regional do Mercado de Embalagens e Rotulagem para Ensaios Clínicos
- A América do Norte dominou o mercado de embalagens e rotulagem para ensaios clínicos, com a maior participação na receita em 2025, impulsionada pelo crescente número de ensaios clínicos, infraestrutura de saúde avançada e adoção cada vez maior de soluções de embalagem centradas no paciente.
- Os prestadores de serviços de saúde e as organizações de pesquisa contratadas (CROs) da região valorizam muito as soluções de embalagem avançadas, em conformidade com as normas e com rastreamento, que melhoram a segurança do paciente, a adesão às regulamentações e a eficiência da cadeia de suprimentos.
- Essa ampla adoção é ainda mais sustentada por fortes investimentos em P&D farmacêutica, estruturas regulatórias que apoiam embalagens inovadoras e a crescente preferência por soluções digitais de rotulagem e serialização em ensaios clínicos.
Análise do Mercado de Embalagens e Rotulagem para Ensaios Clínicos nos EUA
O mercado de embalagens e rotulagem para ensaios clínicos nos EUA detinha a maior participação de receita na América do Norte em 2025, impulsionado pelo crescente número de ensaios clínicos em andamento e pela alta adoção de tecnologias avançadas de embalagem. Os patrocinadores estão priorizando soluções centradas no paciente, incluindo sistemas de embalagem invioláveis, com controle de temperatura e inteligentes. A crescente tendência de ensaios descentralizados e multicêntricos, aliada à integração de soluções digitais como RFID, códigos QR e sistemas de rastreamento, está contribuindo significativamente para a expansão do mercado.
Análise do Mercado Europeu de Embalagens e Rotulagem para Ensaios Clínicos
O mercado europeu de embalagens e rotulagem para ensaios clínicos deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado principalmente por requisitos regulatórios rigorosos e pela crescente demanda por soluções de embalagem seguras e em conformidade com as normas. O aumento no número de ensaios clínicos em oncologia, neurologia e outras áreas terapêuticas está fomentando a adoção de formatos de embalagem especializados. As CROs (Organizações de Pesquisa Clínica) e as empresas farmacêuticas europeias também estão adotando embalagens inteligentes e centradas no paciente para aumentar a eficiência dos ensaios e a integridade dos dados.
Análise do Mercado de Embalagens e Rotulagem para Ensaios Clínicos no Reino Unido
O mercado de embalagens e rotulagem para ensaios clínicos no Reino Unido deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado pela expansão do ecossistema de pesquisa clínica e pelo foco na segurança e adesão do paciente. A ênfase regulatória na serialização, na inviolabilidade e na rotulagem digital está incentivando a adoção dessas tecnologias. Além disso, a robusta infraestrutura de saúde do Reino Unido e a adoção de tecnologias em ensaios clínicos apoiam a crescente implementação de soluções de embalagens automatizadas e inteligentes.
Análise do Mercado de Embalagens e Rotulagem para Ensaios Clínicos na Alemanha
O mercado alemão de embalagens e rotulagem para ensaios clínicos deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado pelo aumento das atividades de ensaios clínicos, pela forte pesquisa e desenvolvimento farmacêutico e pela demanda por soluções de embalagem avançadas, ecológicas e em conformidade com as normas. A ênfase da Alemanha na digitalização, na transparência da cadeia de suprimentos e em abordagens centradas no paciente promove a integração de tecnologias de rotulagem inteligente em ensaios multifásicos.
Análise do Mercado de Embalagens e Rotulagem para Ensaios Clínicos na Região Ásia-Pacífico
O mercado de embalagens e rotulagem para ensaios clínicos na região Ásia-Pacífico deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado pelo aumento dos investimentos em ensaios clínicos, pela expansão da pesquisa e desenvolvimento farmacêutico e pela crescente conscientização sobre tecnologias avançadas de embalagem em países como China, Japão e Índia. A crescente rede de CROs (Organizações de Pesquisa Clínica) da região, aliada a soluções de embalagem com boa relação custo-benefício e a estruturas regulatórias favoráveis, está acelerando a adoção do mercado.
Análise do Mercado Japonês de Embalagens e Rotulagem para Ensaios Clínicos
O mercado japonês de embalagens e rotulagem para ensaios clínicos deverá apresentar o crescimento mais rápido entre 2026 e 2033, devido ao sistema de saúde avançado do país, à adoção de tecnologia e ao foco na segurança do paciente. O número crescente de ensaios clínicos com produtos biológicos e medicina personalizada, aliado à integração de embalagens inteligentes para produtos experimentais sensíveis à temperatura, está impulsionando o crescimento do mercado.
Análise do Mercado de Embalagens e Rotulagem para Ensaios Clínicos na China
O mercado chinês de embalagens e rotulagem para ensaios clínicos representou a maior fatia de receita na região Ásia-Pacífico em 2025, impulsionado pelo crescente investimento em pesquisa e desenvolvimento farmacêutico, pelo rápido aumento no número de ensaios clínicos e pela adoção de tecnologias de rotulagem digital e rastreamento. A busca por soluções de embalagens inteligentes, em conformidade com as normas e centradas no paciente, juntamente com a forte capacidade de produção nacional, está impulsionando o mercado na China.
Participação de mercado em embalagens e rotulagem para ensaios clínicos
O setor de embalagens e rotulagem para ensaios clínicos é liderado principalmente por empresas consolidadas, incluindo:
• Sharp (Reino Unido)
• PCI Pharma Services (EUA)
• MYODERM (Reino Unido)
• Clinigen Group plc (Reino Unido)
• KLIFO (Reino Unido)
• CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (EUA)
• Parexel International Corporation (EUA)
• Alium Medical Limited (Reino Unido)
• Ancillare, LP (EUA)
• Movianto (Reino Unido)
• Bionical Ltd. (Reino Unido)
• Thermo Fisher Scientific Inc. (EUA)
• Catalent, Inc. (EUA)
• Almac Group (Reino Unido)
• Biocair (Reino Unido)
• SIRO Clinpharm Private Limited (Reino Unido)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.