Europe Preclinical Imaging Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
483.94 Million
USD
765.53 Million
2024
2032
USD
483.94 Million
USD
765.53 Million
2024
2032
| 2025 –2032 | |
| USD 483.94 Million | |
| USD 765.53 Million | |
|
|
|
|
Segmentação do mercado de imagens pré-clínicas na Europa, por produto (sistemas e serviços), reagente (reagentes para imagens ópticas pré-clínicas, reagentes para imagens nucleares pré-clínicas, agentes de contraste para ressonância magnética pré-clínica, agentes de contraste para ultrassom pré-clínico e agentes de contraste para tomografia computadorizada pré-clínica), aplicação (pesquisa e desenvolvimento, descoberta de medicamentos, biodistribuição, detecção de células cancerígenas, biomarcadores e outros), usuário final (organização de pesquisa contratada, empresas farmacêuticas e de biotecnologia , institutos de pesquisa acadêmicos e governamentais, centros de diagnóstico e outros) - tendências e previsões do setor até 2032
Tamanho do mercado de imagens pré-clínicas na Europa
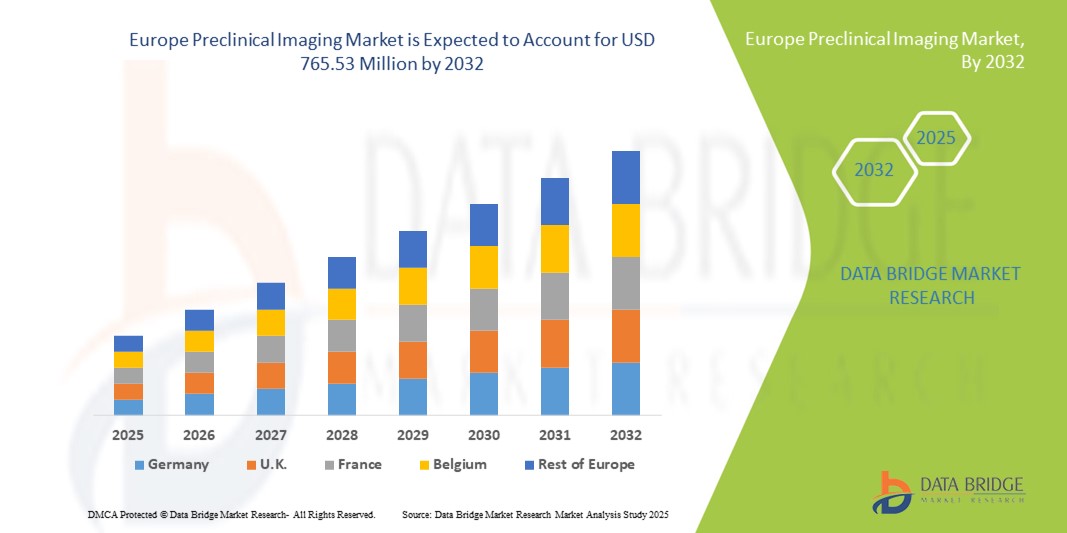
- O tamanho do mercado de imagens pré-clínicas da Europa foi avaliado em US$ 483,94 milhões em 2024 e deve atingir US$ 765,53 milhões até 2032 , com um CAGR de 5,90% durante o período previsto.
- O crescimento do mercado é amplamente impulsionado pela crescente adoção de tecnologias avançadas de imagem na descoberta e desenvolvimento de medicamentos, apoiadas pelo progresso tecnológico em modalidades como PET, ressonância magnética e imagem óptica, levando a uma maior digitalização e precisão na pesquisa pré-clínica.
- Além disso, a crescente demanda por soluções de imagem precisas, não invasivas e de alta resolução entre empresas farmacêuticas e de biotecnologia está consolidando a imagem pré-clínica como uma ferramenta crucial para avaliar a eficácia e a segurança de medicamentos. Esses fatores convergentes estão acelerando a adoção de soluções de imagem pré-clínica, impulsionando significativamente o crescimento do setor.
Análise do Mercado de Imagem Pré-clínica na Europa
- A imagem pré-clínica, envolvendo técnicas de visualização não invasivas, como PET, SPECT, ressonância magnética, tomografia computadorizada, ultrassom e imagem óptica, é um pilar da pesquisa biomédica, permitindo o estudo detalhado de modelos de doenças, desenvolvimento de medicamentos e avaliação terapêutica em múltiplas aplicações.
- A crescente demanda por imagens pré-clínicas é impulsionada principalmente pelo aumento dos investimentos em P&D farmacêutico, pela ênfase crescente na pesquisa translacional e pela necessidade de ferramentas avançadas para avaliar a segurança e a eficácia dos medicamentos em modelos animais antes dos testes em humanos.
- A Alemanha dominou o mercado europeu de imagem pré-clínica, com a maior participação na receita, de 34,6% em 2024, apoiada por sua infraestrutura avançada de pesquisa, alta concentração de empresas farmacêuticas e de biotecnologia e financiamento governamental para inovação em ciências da vida. A sólida rede de instituições acadêmicas de pesquisa e as colaborações com líderes do setor impulsionam ainda mais a adoção de tecnologias avançadas de imagem, como ressonância magnética de alta resolução e sistemas híbridos de PET/RM.
- Espera-se que a França seja o país com crescimento mais rápido no mercado europeu de imagens pré-clínicas durante o período previsto, registrando o maior CAGR de 9,8% entre 2025 e 2032, impulsionado pelo aumento dos investimentos governamentais em pesquisa biomédica, pela expansão das redes de CROs e pela crescente adoção de técnicas de imagem multimodal para pesquisas em oncologia e neurologia. A crescente presença de centros de pesquisa especializados e a adoção de análises de imagens com IA aceleram ainda mais o crescimento do mercado no país.
- O segmento de sistemas dominou o mercado com a maior participação de receita de 65,4% em 2024, principalmente devido à ampla integração de modalidades avançadas de imagem, como PET, ressonância magnética, tomografia computadorizada e imagem óptica em instituições de pesquisa e empresas farmacêuticas.
Escopo do Relatório e Segmentação do Mercado de Imagem Pré-clínica
|
Atributos |
Principais insights do mercado de imagens pré-clínicas |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de imagens pré-clínicas na Europa
Avanços aprimorados por meio da integração de imagens multimodais
- Uma tendência significativa e crescente no mercado europeu de imagens pré-clínicas é a crescente adoção de plataformas de imagens multimodais, que combinam modalidades como PET/RM, PET/TC e SPECT/TC para fornecer insights estruturais, funcionais e moleculares complementares em um único fluxo de trabalho. Essa integração permite estudos pré-clínicos mais abrangentes e precisos.
- Por exemplo, os sistemas PET/MRI permitem aos investigadores capturar imagens de tecidos moles de alta resolução a partir de ressonância magnética, obtendo simultaneamente dados metabólicos e funcionais a partir do PET, tornando-os particularmente valiosos na investigação oncológica e neurológica.
- Plataformas de imagem híbridas em pesquisa pré-clínica estão sendo cada vez mais utilizadas para desenvolvimento de medicamentos e estudos translacionais, pois ajudam a correlacionar processos biológicos no nível molecular com detalhes anatômicos, melhorando a previsibilidade dos resultados terapêuticos.
- A integração de imagens multimodais também reduz a necessidade de múltiplas sessões de imagens e minimiza o estresse em modelos animais, aumentando assim a conformidade ética e melhorando a eficiência do estudo.
- A combinação perfeita de modalidades permite que os pesquisadores investiguem a progressão da doença e a eficácia dos medicamentos de diferentes perspectivas, proporcionando uma compreensão mais holística dos mecanismos biológicos subjacentes.
- Essa tendência em direção a sistemas multimodais integrados está remodelando fundamentalmente os padrões de pesquisa em imagens pré-clínicas. Consequentemente, empresas importantes como Bruker, PerkinElmer e MR Solutions estão se concentrando no desenvolvimento de plataformas híbridas inovadoras com sensibilidade, resolução e recursos de análise de dados aprimorados.
- A demanda por soluções de imagem pré-clínica multimodal está crescendo rapidamente em instituições acadêmicas, empresas farmacêuticas e CROs, à medida que as partes interessadas priorizam cada vez mais a precisão, a eficiência e a relevância translacional na pesquisa pré-clínica.
Dinâmica do mercado de imagens pré-clínicas na Europa
Motorista
Necessidade crescente devido à crescente demanda por imagens não invasivas no desenvolvimento de medicamentos
- A crescente prevalência de doenças crônicas e o crescente fluxo de novos candidatos a medicamentos são os principais impulsionadores da demanda por tecnologias de imagem pré-clínica. Pesquisadores e empresas farmacêuticas dependem fortemente desses sistemas para avaliar a progressão da doença, a eficácia terapêutica e a segurança em modelos animais antes de passar para os ensaios em humanos.
- Por exemplo, em março de 2023, a Bruker lançou uma solução avançada de imagem pré-clínica de PET/CT, projetada para melhorar a sensibilidade e a resolução em aplicações de pesquisa em oncologia e neurologia. Espera-se que tais inovações, por parte de importantes players, impulsionem o crescimento do setor de Imagem Pré-clínica durante o período previsto.
- À medida que os setores farmacêutico e de biotecnologia se expandem, a necessidade de dados de imagem precisos e reprodutíveis em estudos pré-clínicos torna-se cada vez mais crítica. Os sistemas de imagem pré-clínica oferecem vantagens únicas, como monitoramento longitudinal, redução do uso de animais e maior relevância translacional em comparação com as técnicas convencionais.
- Além disso, o foco crescente em medicina de precisão e terapias direcionadas está aumentando a adoção de ferramentas de imagem de alta resolução, como ressonância magnética, tomografia por emissão de pósitrons (PET) e imagens ópticas. Essas tecnologias permitem que os pesquisadores visualizem interações celulares e moleculares, fornecendo insights mais aprofundados sobre os mecanismos dos medicamentos.
- A conveniência das plataformas de imagem multimodais, que combinam insights estruturais e funcionais em um único fluxo de trabalho, está impulsionando sua adoção em pesquisas acadêmicas, CROs e empresas farmacêuticas. A mudança para sistemas híbridos avançados e a crescente disponibilidade de soluções de imagem pré-clínica de fácil utilização estão contribuindo ainda mais para o crescimento geral do mercado.
Restrição/Desafio
Altos custos de equipamentos e acessibilidade limitada
- Um dos principais desafios que restringem o mercado de imagens pré-clínicas é o alto custo de equipamentos avançados de imagem, incluindo PET/RM e sistemas de ressonância magnética de alto campo. Esses dispositivos exigem investimentos de capital significativos, além de infraestrutura especializada e pessoal qualificado, o que limita sua adoção por institutos de pesquisa e laboratórios menores com restrições orçamentárias.
- Por exemplo, os relatórios destacam que o custo dos sistemas híbridos de imagiologia pré-clínica pode atingir milhões de dólares, criando barreiras financeiras para uma implementação generalizada, especialmente nas regiões em desenvolvimento.
- Enfrentar esses desafios relacionados a custos por meio de projetos de sistemas modulares, modelos de leasing de equipamentos e iniciativas de pesquisa colaborativa é crucial para ampliar a acessibilidade. Empresas como a PerkinElmer e a Bruker estão trabalhando ativamente para introduzir sistemas econômicos, mantendo alta precisão e reprodutibilidade.
- Além disso, os requisitos de manutenção e a necessidade de atualizações regulares de software e hardware aumentam as despesas operacionais, desencorajando ainda mais a adoção em ambientes com recursos limitados.
- Embora os players do setor estejam introduzindo soluções de imagem menores, de bancada e portáteis, a custos relativamente mais baixos, a percepção da imagem pré-clínica como uma tecnologia premium ainda dificulta seu uso generalizado. Superar essas barreiras por meio de financiamento governamental, colaborações entre a academia e a indústria e o desenvolvimento de sistemas de imagem mais acessíveis será essencial para o crescimento sustentado do mercado.
Escopo do mercado de imagens pré-clínicas na Europa
O mercado é segmentado com base no produto, reagente, aplicação e usuário final.
- Por produto
Com base no produto, o mercado europeu de imagens pré-clínicas é segmentado em sistemas e serviços. O segmento de sistemas dominou o mercado com a maior participação na receita, de 65,4% em 2024, principalmente devido à ampla integração de modalidades avançadas de imagem, como PET, ressonância magnética, tomografia computadorizada e imagem óptica, em instituições de pesquisa e empresas farmacêuticas. Esses sistemas são essenciais para a realização de estudos longitudinais não invasivos que permitem aos pesquisadores monitorar a progressão da doença e a resposta ao tratamento ao longo do tempo. Sua capacidade de fornecer imagens de alta resolução, combinada com recursos multimodais, os torna indispensáveis em oncologia, neurologia e pesquisa cardiovascular. As empresas farmacêuticas também dependem de sistemas de imagens pré-clínicas para reduzir as taxas de falha em ensaios clínicos, obtendo insights mais profundos na fase pré-clínica. Os contínuos avanços tecnológicos em sistemas híbridos de imagem, juntamente com o forte ecossistema de P&D da Europa, fortalecem ainda mais o domínio desse segmento. Além disso, investimentos significativos em infraestrutura de imagem na Alemanha, França e Reino Unido reforçam sua liderança de mercado.
Espera-se que o segmento de serviços registre o CAGR mais rápido de 8,9% de 2025 a 2032, impulsionado pela crescente demanda por terceirização de estudos complexos de imagem para provedores de serviços especializados e CROs. Com o aumento dos custos e os desafios técnicos associados à operação de sistemas avançados de imagem, muitas empresas farmacêuticas e de biotecnologia preferem colaborar com provedores de serviços para acessar tecnologia de ponta sem grandes investimentos de capital. CROs e instituições acadêmicas na Europa estão cada vez mais oferecendo serviços de imagem pré-clínica de ponta a ponta, incluindo desenho de estudo, análise de dados e suporte regulatório. Essa tendência é especialmente prevalente entre pequenas e médias empresas de biotecnologia que não possuem infraestrutura interna. A flexibilidade de serviços personalizados e o acesso a profissionais especialistas em imagem tornam esse segmento ainda mais atraente. Além disso, parcerias estratégicas entre CROs e empresas farmacêuticas estão acelerando a adoção, alimentando um forte potencial de crescimento para esse segmento nos próximos anos.
- Por reagente
Com base no reagente, o mercado europeu de imagens pré-clínicas é segmentado em reagentes de imagem óptica pré-clínica, reagentes de imagem nuclear pré-clínica, agentes de contraste pré-clínicos para ressonância magnética, agentes de contraste pré-clínicos para ultrassom e agentes de contraste pré-clínicos para tomografia computadorizada. O segmento de reagentes de imagem óptica pré-clínica dominou o mercado com uma participação de receita de 37,8% em 2024, devido à sua ampla aplicação na visualização de processos moleculares e celulares com alta sensibilidade e especificidade. Reagentes ópticos, como sondas bioluminescentes e fluorescentes, são amplamente utilizados em biologia do câncer, análise de expressão gênica e estudos de eficácia de medicamentos. Sua relação custo-benefício e facilidade de uso, em comparação com outros agentes de imagem, os tornam altamente acessíveis para instituições de pesquisa acadêmicas e comerciais. Além disso, os reagentes ópticos permitem imagens não invasivas de organismos vivos em tempo real, o que aumenta significativamente a precisão e a eficiência da pesquisa. A ampla adoção de reagentes de imagem óptica em toda a Europa, particularmente em projetos acadêmicos e financiados pelo governo, consolida sua posição como o segmento dominante. A inovação contínua no desenvolvimento de sondas, como marcadores fluorescentes direcionados, reforça ainda mais sua crescente utilidade na pesquisa pré-clínica.
O segmento de reagentes para imagem nuclear pré-clínica deverá apresentar o CAGR mais rápido, de 9,7%, entre 2025 e 2032, apoiado pelo uso crescente das tecnologias PET e SPECT para aplicações avançadas de desenvolvimento de medicamentos. Os reagentes nucleares fornecem recursos de imagem quantitativa e penetração profunda em tecidos, tornando-os particularmente valiosos em oncologia, neurologia e pesquisa metabólica. O uso crescente de radiotraçadores para estudos de biodistribuição e farmacocinética oferece insights precisos sobre o desempenho de medicamentos em estágios iniciais. A forte infraestrutura radiofarmacêutica da Europa, especialmente em países como Alemanha e França, está acelerando a adoção de reagentes para imagem nuclear. Além disso, as colaborações entre empresas farmacêuticas e centros de pesquisa em medicina nuclear estão expandindo o acesso a radiotraçadores inovadores. A crescente demanda por medicina personalizada e pesquisa em oncologia de precisão também está impulsionando o crescimento do segmento, tornando-o uma das áreas mais dinâmicas do mercado europeu de imagem pré-clínica.
- Por aplicação
Com base na aplicação, o mercado europeu de imagens pré-clínicas é segmentado em pesquisa e desenvolvimento, descoberta de fármacos, biodistribuição, detecção de células cancerígenas, biomarcadores e outros. O segmento de pesquisa e desenvolvimento deteve a maior fatia de mercado, com 41,5% de receita em 2024, impulsionado principalmente por investimentos substanciais em P&D de instituições farmacêuticas, de biotecnologia e acadêmicas em toda a Europa. A imagem pré-clínica desempenha um papel crítico na compreensão dos mecanismos da doença, na validação de alvos terapêuticos e no monitoramento dos resultados do tratamento nos estágios iniciais do desenvolvimento de fármacos. Sua capacidade de fornecer dados precisos e não invasivos em tempo real a torna indispensável para os esforços de pesquisa translacional. O crescente foco na medicina de precisão, combinado com a forte rede europeia de programas de pesquisa acadêmica e governamental, fortalece ainda mais o domínio desse segmento. Instituições de pesquisa na Alemanha, Reino Unido e França estão utilizando cada vez mais sistemas avançados de imagem para melhorar a produtividade da pesquisa e acelerar os cronogramas de desenvolvimento de fármacos. A liderança do segmento também é reforçada por iniciativas de financiamento da UE e de governos nacionais que incentivam a P&D baseada em imagens.
Espera-se que o segmento de descoberta de medicamentos registre o CAGR mais rápido de 10,3% de 2025 a 2032, impulsionado pela crescente ênfase na aceleração do desenvolvimento de novas terapias para câncer, distúrbios neurológicos e doenças raras. As tecnologias de imagem pré-clínica permitem que os pesquisadores monitorem as interações fármaco-alvo, a biodistribuição e a eficácia terapêutica em modelos vivos, reduzindo significativamente o tempo e o custo da descoberta de medicamentos. A demanda por soluções de imagem de alto rendimento e alta resolução está aumentando à medida que as empresas farmacêuticas migram para produtos biológicos e terapias baseadas em células. As empresas biofarmacêuticas europeias estão alavancando a imagem para agilizar os processos de tomada de decisão em pipelines de medicamentos em estágio inicial. Além disso, os avanços em plataformas de imagem multimodais que combinam PET, RNM e modalidades ópticas fornecem insights aprimorados, tornando-as ferramentas valiosas para a descoberta de medicamentos. A forte colaboração entre a indústria e a academia acelera ainda mais a inovação, posicionando este segmento como o de crescimento mais rápido na região.
- Por usuário final
Com base no usuário final, o mercado europeu de imagens pré-clínicas é segmentado em organizações de pesquisa contratadas (CROs), empresas farmacêuticas e de biotecnologia, institutos de pesquisa acadêmicos e governamentais, centros de diagnóstico e outros. O segmento de empresas farmacêuticas e de biotecnologia dominou o mercado com uma participação de 44,6% em 2024, refletindo a dependência do setor em tecnologias de imagens pré-clínicas para apoiar os pipelines de desenvolvimento de medicamentos. Essas empresas investem fortemente em sistemas de imagens para conduzir ensaios pré-clínicos que garantem eficácia, segurança e conformidade regulatória. A imagem fornece visualização em tempo real dos efeitos dos medicamentos, o que é vital para o desenvolvimento de produtos biológicos complexos, terapias genéticas e medicamentos de precisão. A presença de importantes polos farmacêuticos na Alemanha, Suíça e Reino Unido reforça ainda mais a força do segmento. Além disso, a crescente ênfase em excipientes à base de plantas e rótulos limpos em formulações de medicamentos está indiretamente aumentando a demanda por imagens para garantia de qualidade. As empresas farmacêuticas também estão expandindo parcerias com fornecedores de imagens e CROs para fortalecer a eficiência de P&D, consolidando sua posição dominante no mercado.
Espera-se que o segmento de organizações de pesquisa contratadas (CROs) registre o CAGR mais rápido de 11,2% de 2025 a 2032, à medida que as empresas farmacêuticas e de biotecnologia terceirizam cada vez mais estudos de imagem pré-clínica para otimizar custos e acessar conhecimento especializado. As CROs fornecem soluções abrangentes de imagem, incluindo desenho de estudo, imagem multimodal e análise avançada de dados, que geralmente são mais econômicas do que desenvolver recursos internos. Essa tendência é particularmente forte entre pequenas e médias empresas de biotecnologia que não têm recursos para estabelecer instalações dedicadas de imagem. Na Europa, as CROs estão expandindo seus portfólios de serviços integrando análise de imagem baseada em IA e plataformas em nuvem, aumentando a eficiência e a escalabilidade. Parcerias estratégicas entre CROs e grandes empresas farmacêuticas também estão criando novas vias de crescimento. À medida que os requisitos regulatórios para validação pré-clínica se tornam mais rigorosos, a terceirização de serviços de imagem para CROs garante a conformidade e, ao mesmo tempo, reduz os prazos, tornando esse segmento a categoria de usuário final que mais cresce.
Análise regional do mercado de imagens pré-clínicas na Europa
- O mercado europeu de imagens pré-clínicas deverá expandir-se a um CAGR substancial ao longo do período previsto, impulsionado principalmente pela crescente procura de tecnologias avançadas de imagem na descoberta de medicamentos e na investigação biomédica.
- A crescente prevalência de doenças crônicas, o crescimento do desenvolvimento de produtos biológicos e a necessidade de ferramentas de pesquisa translacional estão fomentando a adoção de sistemas de imagem pré-clínica em toda a região. A sólida base de empresas farmacêuticas da Europa, aliada a uma robusta infraestrutura acadêmica e de pesquisa clínica, está apoiando o desenvolvimento e o uso de modalidades como PET, ressonância magnética, tomografia computadorizada e imagem óptica.
- Além disso, o financiamento governamental e os projetos colaborativos entre a indústria e a academia estão acelerando a integração de plataformas de imagem multimodal e de alta resolução. A região está vivenciando um forte crescimento em aplicações de pesquisa em oncologia, neurologia e cardiologia, com a imagem pré-clínica sendo cada vez mais utilizada para melhorar a eficiência, a precisão e a previsibilidade em processos de desenvolvimento de medicamentos.
Visão do mercado de imagens pré-clínicas na Alemanha
O mercado alemão de imagens pré-clínicas dominou o mercado europeu de imagens pré-clínicas, com a maior participação na receita, de 34,6% em 2024, apoiado por sua infraestrutura avançada de pesquisa, alta concentração de empresas farmacêuticas e de biotecnologia e financiamento governamental para inovação em ciências da vida. A forte rede de instituições acadêmicas de pesquisa e as colaborações com líderes do setor impulsionam ainda mais a adoção de tecnologias avançadas de imagem, como ressonância magnética de alta resolução e sistemas híbridos de PET/RM. A demanda é particularmente forte em pesquisas oncológicas e neurológicas, onde a Alemanha é um polo líder em estudos translacionais. Além disso, a presença de fabricantes europeus de equipamentos de imagem e o crescente investimento em análises de imagem baseadas em IA contribuem para sua posição de liderança na região.
Visão do mercado de imagens pré-clínicas na França
Espera-se que o mercado francês de imagens pré-clínicas seja o de crescimento mais rápido na Europa durante o período previsto, registrando o maior CAGR de 9,8% entre 2025 e 2032, impulsionado pelo aumento dos investimentos governamentais em pesquisa biomédica, pela expansão das redes de CROs e pela crescente adoção de técnicas de imagem multimodal para pesquisa em oncologia e neurologia. A crescente presença de centros de pesquisa especializados no país, juntamente com parcerias público-privadas ativas, está alimentando a demanda por ferramentas avançadas de imagem pré-clínica. Além disso, a adoção de análise de imagem habilitada por IA e plataformas híbridas inovadoras está melhorando a eficiência dos estudos pré-clínicos. Esses fatores, combinados com a forte ênfase da França em inovação biomédica de ponta, posicionam-na como o mercado de crescimento mais rápido na Europa.
Participação no mercado de imagens pré-clínicas na Europa
O setor de imagens pré-clínicas é liderado principalmente por empresas bem estabelecidas, incluindo:
- PerkinElmer (EUA)
- FUJIFILM VisualSonics, Inc. (Canadá)
- Bruker (EUA)
- LI-COR, Inc. (EUA)
- Aspect Imaging Ltda. (Israel)
- Berthold Technologies GmbH & Co. KG (Alemanha)
- MILabs BV (Holanda)
- Trifoil Imaging LLC (EUA)
- Mediso Ltd. (Hungria)
- IVIM Technology Inc. (Coreia do Sul)
- Soluções MR (Reino Unido)
- Photon etc. (Canadá)
- Siemens Healthineers AG (Alemanha)
- GE Healthcare (Reino Unido)
- Koninklijke Philips NV, (Holanda)
- CANON MEDICAL SYSTEMS CORPORATION (Japão)
- NIKON CORPORATION (Japão)
- Olympus Corporation (Japão)
- Grupo Zeiss (Alemanha)
- Leica Microsystems (Alemanha)
- Thermo Fisher Scientific Inc. (EUA)
- Agilent Technologies, Inc. (EUA)
Últimos desenvolvimentos no mercado de imagens pré-clínicas na Europa
- Em abril de 2025, a Revvity, Inc. apresentou o Sistema de Injeção Guiada por Imagem VivoJect na Reunião Anual da Associação Americana para Pesquisa do Câncer (AACR), em Chicago. Este sistema, em conjunto com o sistema de ultrassom pré-clínico automatizado Vega, aprimora a geração de imagens em tempo real e injeções precisas para estudos in vivo de alto rendimento. Ele agiliza os fluxos de trabalho no desenvolvimento de modelos tumorais, administração direcionada de medicamentos, terapia gênica, pesquisa com células-tronco e estudos cardíacos.
- Em maio de 2025, a MR Solutions instalou o primeiro sistema trimodal de SPECT/RM 7T de alto campo do mundo no Houston Methodist. Esta instalação destaca a inovação contínua em imagens pré-clínicas, possibilitando recursos abrangentes de imagem para aplicações avançadas de pesquisa.
- Em fevereiro de 2024, a Bruker Corporation adquiriu a Spectral Instruments Imaging LLC, aprimorando seu portfólio de produtos no setor de imagem pré-clínica. Esta aquisição expande a gama de soluções pré-clínicas da Bruker, especialmente na pesquisa de doenças, em linha com a crescente demanda por tecnologias avançadas de imagem.
- Em julho de 2024, o MILabs atualizou seu sistema U-CT para imagens in vivo de modelos animais com COVID-19. Essa atualização fornece imagens pulmonares não invasivas de ultra-alta resolução, permitindo a localização precisa de processos patológicos em pequenos animais, promovendo assim a pesquisa em doenças infecciosas.
- Em setembro de 2023, a Revvity, Inc. expandiu seu portfólio de imagens pré-clínicas com a introdução de sistemas avançados, como os sistemas de imagem IVIS SpectrumCT 2, a solução de imagem Quantum GX3 microCT e o sistema de ultrassom pré-clínico Vega. Esses sistemas possibilitam descobertas revolucionárias na pesquisa pré-clínica, oferecendo recursos aprimorados de imagem para diversas aplicações.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.