Europe Hla Typing Transplant Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
194.35 Million
USD
344.87 Million
2020
2028
USD
194.35 Million
USD
344.87 Million
2020
2028
| 2021 –2028 | |
| USD 194.35 Million | |
| USD 344.87 Million | |
|
|
|
Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market, By Products and Services (Reagents and Consumables, Instruments, Software and Services), Technology (Sequencing- Based Molecular Assays, Molecular Assay Technologies, Non- Molecular Assay Technologies), Transplant Type (Solid Organ Transplant, Haematopoietic Stem Cell Transplant), Application (Diagnostic Applications, Research Application), End User (Independent Reference Laboratories, Hospitals and Transplant Centres, Research Laboratories and Academic Institutes), Country (U.K., France, Italy, Spain, Switzerland, Russia, Netherlands, Turkey, Belgium, and Rest of Europe) Industry Trends and Forecast to 2028.
 Market Analysis and Insights: Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market
Market Analysis and Insights: Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market
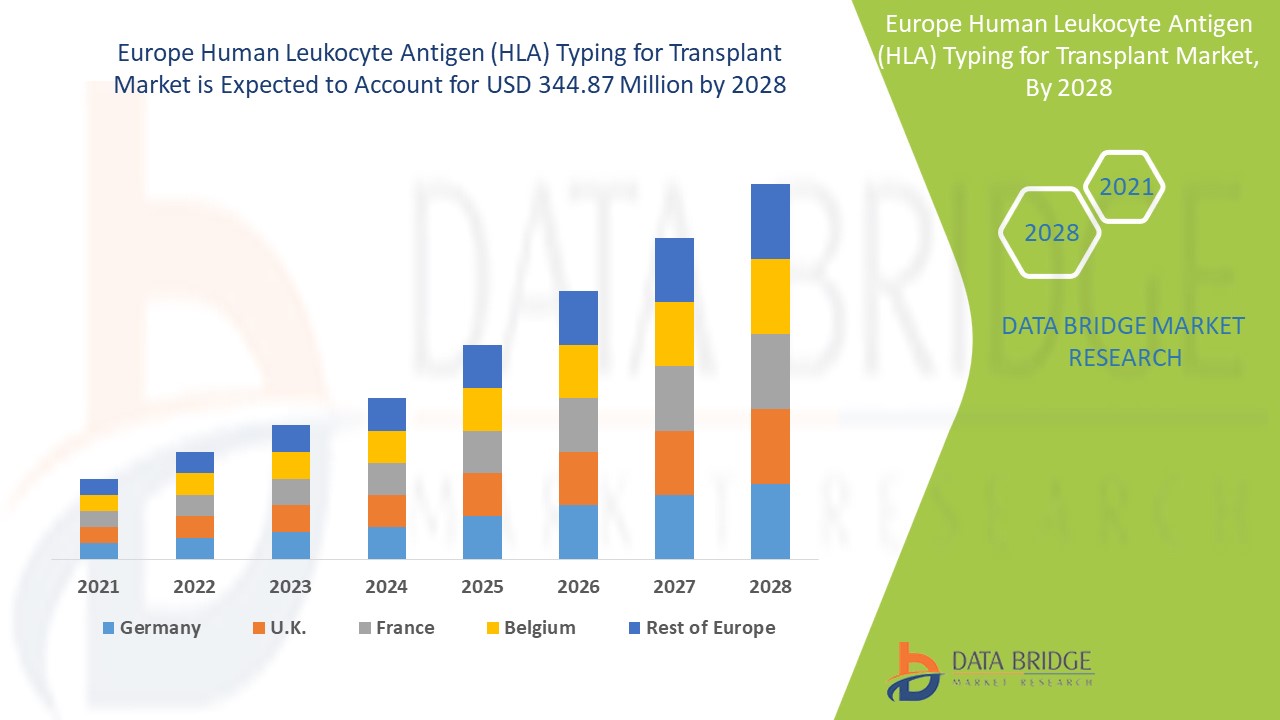
Europe human leukocyte antigen (HLA) typing for transplant market is expected to gain significant growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the Europe human leukocyte antigen (HLA) typing for transplant market is growing with a CAGR of 7.8% in the forecast period of 2021 to 2028 and is expected to reach USD 344.87 million by 2028 from USD 194.35 million in 2020. Rising demand for organ transplant procedures is acting as driver for the growth of global human leukocyte typing for transplant market.
Human leukocyte antigen (HLA) typing is used to match patients and donors for bone marrow or cord blood transplants. HLA are proteins -- or markers -- found on most cells in the body. The immune system uses these markers to recognize which cells belong to the body and which do not.
Research has found that a donor must match a minimum of 6 HLA markers. Several Many times a closer match is required. A best match is found through detailed testing. As some HLA types are more common than others, some patients may face a greater challenge in finding a matching donor. Some HLA types are found more often in certain racial and ethnic groups.
A close match between a donor’s and a patient’s HLA markers is essential for a successful transplant outcome. HLA matching promotes the growth and development of new healthy blood cells (called engraftment) and reduces the risk of a post-transplant complication called graft-versus-host (GVHD) disease.
The high demand of organ donation, increasing innovations and technologies and novel product launch are also propelling the growth of the Europe human leukocyte antigen (HLA) typing for transplant market.
A growing number of people are affected with by the HLA disorders which demand highly effective and advanced treatment to minimize the risk during the treatment. The healthcare systems need highly advanced drugs for numerous different types of HLA disorders during the treatment of patients. Therefore, the major market players are highly focusing on product launches and product approvals. Additionally, the government and regulatory bodies are supporting market players by virtue of product approval.
Rising product launches is acting as an opportunity for this market and is expected to impel the market growth for boosting the demand of global human leukocyte typing for transplant market.
Research on the human leukocyte antigen (HLA), is an extensively studied molecule involved in immunity and NGS technologies have revolutionised HLA typing procedure by providing fast and cost-effective technology. It is expected that lack skilled professionals is the major key challenge for the future growth of global human leukocyte antigen (HLA) typing for transplant market.
Europe human leukocyte antigen (HLA) typing for transplant market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
 Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market and Market Size
Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market and Market Size
Europe human leukocyte antigen (HLA) typing for transplant market is segmented on the basis of products and services, technology, transplant type, application, and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of by products and services, Europe human leukocyte antigen (HLA) typing for transplant market is segmented in reagents and consumables, instruments, software and services. In 2021, instruments segment is expected to dominate the market due to high demand for organ transplantation that has rapidly increased.
- On the basis of technology, Europe human leukocyte antigen (HLA) typing for transplant market is molecular assay technologies and non- molecular assay technologies. In 2021, molecular assay technologies segment is expected to dominate the market due to rising awareness about the disease.
- On the basis of transplant type, Europe human leukocyte antigen (HLA) typing for transplant market is segmented solid organ transplant and haematopoietic stem cell transplant. In 2021, solid organ transplant segment is expected to dominate the market because of increasing prevalence of this disease type among target population.
- On the basis of application, the Europe human leukocyte antigen (HLA) typing for transplant market is segmented into diagnostic applications and research application. In 2021, diagnostic applications segment is expected to dominate the market because of high pool of patients with cancer and genetic diseases.
- On the basis of end user, the Europe human leukocyte antigen (HLA) typing for transplant market is segmented into independent reference laboratories, hospitals and transplant centres, research laboratories and academic institutes. In 2021, hospitals & transplant centers segment is expected to dominate the market owing to the rising product launches by the key market players.
Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market Country Level Analysis
The Europe human leukocyte antigen (HLA) typing for transplant market is analyzed and market size information is provided based on country, products and services, technology, transplant type, application, and end user.
Countries covered in the Europe human leukocyte antigen (HLA) typing for transplant market report are Germany, France, U.K., Hungary, Italy, Spain, Russia, Turkey, Belgium, Netherlands, Switzerland, and rest of Europe.
U.K. is expected to dominate the Europe human leukocyte antigen (HLA) typing for transplant market due to increasing rising government funding in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Rising Healthcare Expenditure and Escalation in Innovations and Technologies in the Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market are Creating New Opportunities for Players in the Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market
Europe human leukocyte antigen (HLA) typing for transplant market also provides you with detailed market analysis for every country growth in particular industry with the Europe human leukocyte antigen (HLA) typing for transplant market sales, impact of advancement in the Europe human leukocyte antigen (HLA) typing for transplant market and changes in regulatory scenarios with their support for the Europe human leukocyte antigen (HLA) typing for transplant market in the period of 2011-2019.
Competitive Landscape and Europe Human Leukocyte Antigen (HLA) Typing for Transplant Market Share Analysis
Europe human leukocyte antigen (HLA) typing for transplant market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, and technology lifeline curve. The above data points provided are only related to the companies’ focus related to Europe human leukocyte antigen (HLA) typing for transplant market.
Major players covered in the report are Thermo Fisher Scientific Inc., Illumina, Inc., Luminex Corporation, QIAGEN, CareDX, Inc., Immucor Inc., Bio Rad Labaratories, Inc., Takara Bio Inc., BD, Fujirebio, TBG Diagnostics Limited, and GenDx.
DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Many product launches and agreements are also initiated by the companies worldwide, which are further accelerating the Europe human leukocyte antigen (HLA) typing for transplant market.
For instance,
- In October 2017, TBG's HLAssure SE SBT Kits received the China CFDA approval for clinical use. This is the first approval for a High Resolution HLA Typing Kit in China. This helped the company to strengthened its footprint in China and boosted market growth
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT & SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 AVERAGE PRICE/TEST FOR HLA TYPING BY NEXT GENERATION SEQUENCING (USD)
4.2 PESTEL'S MODEL
4.3 PORTER'S 5 FORCES
5 REGIONAL SUMMARY
5.1 EUROPE
5.1.1 OVERVIEW
6 REGULATORY GUIDELINES FOR EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) FOR TRANSPLANT MARKET
6.1 U.S.
6.2 EUROPE
6.3 JAPAN
6.4 INDIA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING DEMAND FOR ORGAN TRANSPLANT PROCEDURES
7.1.2 GROWING TECHNOLOGICAL ADVANCEMENT & INNOVATIONS IN HLA TYPING
7.1.3 RISING RESEARCH AND DEVELOPMENT ACTIVITIES IN HLA TYPING
7.1.4 INCREASING CERTAIN DISEASES SUCH AS BLOOD CANCERS, AND GENETIC BLOOD DISORDERS
7.2 RESTRAINTS
7.2.1 HIGH COST OF HLA TYPING FOR TRANSPLANTATION PROCEDURE
7.2.2 LIMITED REIMBURSEMENTS POLICIES FOR ORGAN TRANSPLANT
7.3 OPPORTUNITIES
7.3.1 PRODUCT LAUNCHES, AND AGREEMENT BY MAJOR PLAYERS
7.3.2 RISING GOVERNMENT FUNDING FOR ORGAN DONATIONS
7.3.3 RISING PUBLIC AWARENESS FOR ORGAN TRANSPLANTATION
7.4 CHALLENGES
7.4.1 LESS NUMBER OF DONORS FOR ORGAN DONATION
7.4.2 DEARTH OF SKILLED PROFESSIONALS
8 COVID-19 IMPACT ON HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET
8.1 IMPACT ON PRICE
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC DECISIONS FOR MANUFACTURERS
8.5 CONCLUSION
9 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES
9.1 OVERVIEW
9.2 INSTRUMENTS
9.3 REAGENTS AND CONSUMABLES
9.4 SOFTWARE & SERVICES
10 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY
10.1 OVERVIEW
10.2 MOLECULAR ASSAY TECHNOLOGIES
10.2.1 SEQUENCING-BASED MOLECULAR ASSAYS
10.2.1.1 NEXT-GENERATION SEQUENCING
10.2.1.2 SANGER SEQUENCING
10.2.1.3 OTHER SEQUENCING-BASED MOLECULAR ASSAYS
10.2.2 PCR-BASED MOLECULAR ASSAYS
10.2.2.1 SEQUENCE-SPECIFIC PRIMER-PCR
10.2.2.2 REAL-TIME PCR
10.2.2.3 SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR
10.2.2.4 OTHER PCR-BASED MOLECULAR ASSAYS
10.3 NON-MOLECULAR ASSAY TECHNOLOGIES
11 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE
11.1 OVERVIEW
11.2 SOLID ORGAN TRANSPLANT
11.2.1 KIDNEY
11.2.2 LIVER
11.2.3 HEART
11.2.4 LUNG
11.2.5 PANCREAS
11.2.6 OTHERS
11.3 HEMATOPOIETIC STEM CELL TRANSPLANT
11.3.1 STEM CELL TRANSPLANT
11.3.2 BONE MARROW TRANSPLANT
12 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 DIAGNOSTIC APPLICATIONS
12.2.1 ANTIBODY SCREENING
12.2.2 CHIMERISM MONITORING
12.2.3 OTHERS
12.3 RESEARCH APPLICATIONS
13 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS & TRANSPLANT CENTERS
13.3 RESEARCH LABORATORIES & ACADEMIC INSTITUTES
13.4 INDEPENDENT REFERENCE LABORATORIES
14 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 U.K.
14.1.3 FRANCE
14.1.4 ITALY
14.1.5 SPAIN
14.1.6 SWITZERLAND
14.1.7 RUSSIA
14.1.8 NETHERLANDS
14.1.9 TURKEY
14.1.10 BELGIUM
14.1.11 REST OF EUROPE
15 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 MARKET SHARE ANALYSIS: BY TECHNOLOGY
16.1 SEQUENCING BASED MOLECULAR ASSAYS
16.1.1 NORTH AMERICA
16.1.2 EUROPE
16.1.3 ASIA-PACIFIC
16.1.4 SOUTH AMERICA
16.1.5 MIDDLE EAST AND AFRICA
16.2 NGS SEQUENCING
16.2.1 NORTH AMERICA
16.2.2 EUROPE
16.2.3 ASIA-PACIFIC
16.2.4 SOUTH AMERICA
16.2.5 MIDDLE EAST AND AFRICA
16.3 SANGER SEQUENCING
16.3.1 NORTH AMERICA
16.3.2 EUROPE
16.3.3 ASIA-PACIFIC
16.3.4 SOUTH AMERICA
16.3.5 MIDDLE EAST AND AFRICA
16.4 PCR BASED MOLECULAR ASSAYS
16.4.1 NORTH AMERICA
16.4.2 EUROPE
16.4.3 ASIA-PACIFIC
16.4.4 SOUTH AMERICA
16.4.5 MIDDLE EAST AND AFRICA
16.5 REAL TIME PCR
16.5.1 NORTH AMERICA
16.5.2 EUROPE
16.5.3 ASIA-PACIFIC
16.5.4 SOUTH AMERICA
16.5.5 MIDDLE EAST AND AFRICA
16.6 SEQUENCE-SPECIFIC PRIMER-PCR
16.6.1 NORTH AMERICA
16.6.2 EUROPE
16.6.3 ASIA-PACIFIC
16.6.4 SOUTH AMERICA
16.6.5 MIDDLE EAST AND AFRICA
16.7 SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR
16.7.1 NORTH AMERICA
16.7.2 EUROPE
16.7.3 ASIA-PACIFIC
16.7.4 SOUTH AMERICA
16.7.5 MIDDLE EAST AND AFRICA
17 COMPANY PROFILE
17.1 THERMO FISHER SCIENTIFIC INC.
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.1.6 SWOT ANALYSIS
17.2 ILLUMINA, INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.2.6 SWOT ANALYSIS
17.3 BIO-RAD LABORATORIES, INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.3.6 SWOT ANALYSIS
17.4 QIAGEN
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.4.5.1 ACQUISITION
17.4.6 SWOT ANALYSIS
17.5 ROCHE SEQUENCING (A SUBSIDIARY OF F.HOFFMAN LA-ROCHE LTD.)
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.5.6 SWOT ANALYSIS
17.6 BD
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENTS
17.7 BIOFORTUNA LIMITED
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 CARE DX, INC.
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENTS
17.9 CREATIVE BIOLABS
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.9.3.1 PRODUCT LAUNCH/UPDATE/APPROVAL
17.1 FUJIREBIO
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.10.4.1 CE CERTIFICATION
17.11 GENDX
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENTS
17.12 IMMUCOR, INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.12.3.1 PROUCT LAUNCH
17.13 LUMINEX CORPORATION
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENTS
17.14 OMIXON INC.
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.14.3.1 PRODUCT LAUNCH
17.15 TAKARA BIO INC
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENTS
17.15.4.1 LICENSING
17.15.5 PRODUCT LAUNCH
17.16 TBG DIAGNOSTICS LIMITED
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOLIO
17.16.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tabela
TABLE 1 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, AVERAGE PRICE/TEST FOR HLA TYPING BY NEXT GENERATION SEQUENCING
TABLE 2 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BYPRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 3 EUROPE INSTRUMENTS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 4 EUROPE REAGENTS AND CONSUMABLES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 5 EUROPE SOFTWARE & SERVICES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 6 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 7 EUROPE MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 8 EUROPE MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY , 2019-2028 (USD MILLION)
TABLE 9 EUROPE SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY , 2019-2028 (USD MILLION)
TABLE 10 EUROPE PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 11 EUROPE NON-MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 12 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 13 EUROPE SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 14 EUROPE SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE , 2019-2028 (USD MILLION)
TABLE 15 EUROPE HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 16 EUROPE HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 17 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 18 EUROPE DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, REGION, 2019-2028 (USD MILLION)
TABLE 19 EUROPE DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 20 EUROPE RESEARCH APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 21 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 22 EUROPE HOSPITALS & TRANSPLANT CENTERS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 23 EUROPE RESEARCH LABORATORIES & ACADEMIC INSTITUTES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 24 EUROPE OTHERS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 25 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY COUNTRY, 2019-2028 (USD MILLION)
TABLE 26 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 27 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 28 EUROPE MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 29 EUROPE SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 30 EUROPE PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 31 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 32 EUROPE SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 33 EUROPE HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 34 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 35 EUROPE DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 36 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 37 GERMANY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 38 GERMANY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 39 GERMANY MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 40 GERMANY SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 41 GERMANY PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 42 GERMANY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 43 GERMANY SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 44 GERMANY HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 45 GERMANY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 46 GERMANY DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 47 GERMANY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 48 U.K. HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 49 U.K. HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 50 U.K. MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 51 U.K. SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 52 U.K. PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 53 U.K. HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 54 U.K. SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 55 U.K. HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 56 U.K. HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 57 U.K. DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 58 U.K. HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 59 FRANCE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 60 FRANCE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 61 FRANCE MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 62 FRANCE SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 63 FRANCE PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 64 FRANCE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 65 FRANCE SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 66 FRANCE HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 67 FRANCE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 68 FRANCE DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 69 FRANCE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 70 ITALY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 71 ITALY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 72 ITALY MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 73 ITALY SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 74 ITALY PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 75 ITALY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 76 ITALY SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 77 ITALY HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 78 ITALY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 79 ITALY DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 80 ITALY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 81 SPAIN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 82 SPAIN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 83 SPAIN MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 84 SPAIN SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 85 SPAIN PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 86 SPAIN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 87 SPAIN SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 88 SPAIN HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 89 SPAIN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 90 SPAIN DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 91 SPAIN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 92 SWITZERLAND HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 93 SWITZERLAND HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 94 SWITZERLAND MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 95 SWITZERLAND SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 96 SWITZERLAND PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 97 SWITZERLAND HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 98 SWITZERLAND SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 99 SWITZERLAND HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 100 SWITZERLAND HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 101 SWITZERLAND DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 102 SWITZERLAND HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 103 RUSSIA HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 104 RUSSIA HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 105 RUSSIA MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 106 RUSSIA SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 107 RUSSIA PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 108 RUSSIA HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 109 RUSSIA SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 110 RUSSIA HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 111 RUSSIA HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 112 RUSSIA DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 113 RUSSIA HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 114 NETHERLANDS HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 115 NETHERLANDS HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 116 NETHERLANDS MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 117 NETHERLANDS SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 118 NETHERLANDS PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 119 NETHERLANDS HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 120 NETHERLANDS SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 121 NETHERLANDS HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 122 NETHERLANDS HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 123 NETHERLANDS DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 124 NETHERLANDS HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 125 TURKEY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 126 TURKEY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 127 TURKEY MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 128 TURKEY SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 129 TURKEY PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 130 TURKEY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 131 TURKEY SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 132 TURKEY HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 133 TURKEY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 134 TURKEY DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 135 TURKEY HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 136 BELGIUM HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
TABLE 137 BELGIUM HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 138 BELGIUM MOLECULAR ASSAY TECHNOLOGIES IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 139 BELGIUM SEQUENCING-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 140 BELGIUM PCR-BASED MOLECULAR ASSAYS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TECHNOLOGY, 2019-2028 (USD MILLION)
TABLE 141 BELGIUM HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 142 BELGIUM SOLID ORGAN TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 143 BELGIUM HEMATOPOIETIC STEM CELL TRANSPLANT IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY TRANSPLANT TYPE, 2019-2028 (USD MILLION)
TABLE 144 BELGIUM HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 145 BELGIUM DIAGNOSTIC APPLICATIONS IN HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 146 BELGIUM HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY END USERS, 2019-2028 (USD MILLION)
TABLE 147 REST OF EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, BY PRODUCT & SERVICES, 2019-2028 (USD MILLION)
Lista de Figura
FIGURE 1 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: SEGMENTATION
FIGURE 2 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET:
FIGURE 3 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: DROC ANALYSIS
FIGURE 4 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: SEGMENTATION
FIGURE 11 INCREASING DEMAND FOR ORGAN TRANSPLANTATION, AND RISING R&D ACTIVITIES IN HLA TYPING ARE EXPECTED TO DRIVE THE EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 12 INSTRUMENTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET IN 2021 & 2028
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET, AND ASIA-PACIFIC EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 14 ASIA-PACIFIC IS THE FASTEST GROWING MARKET FOR HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MANUFACTURERS IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET
FIGURE 16 IN 2021, ESTIMATED NEW CASES OF LEUKEMIA, MYELOMA, AND LYMPHOMA IN THE U.S.
FIGURE 17 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY PRODUCTS & SERVICES, 2020
FIGURE 18 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY PRODUCTS & SERVICES, 2021-2028 (USD MILLION)
FIGURE 19 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY PRODUCTS & SERVICES, CAGR (2021-2028)
FIGURE 20 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY PRODUCTS & SERVICES, LIFELINE CURVE
FIGURE 21 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TECHNOLOGY, 2020
FIGURE 22 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TECHNOLOGY, 2021-2028 (USD MILLION)
FIGURE 23 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TECHNOLOGY, CAGR (2021-2028)
FIGURE 24 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 25 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TRANSPLANT TYPE, 2020
FIGURE 26 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TRANSPLANT TYPE, 2021-2028 (USD MILLION)
FIGURE 27 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TRANSPLANT TYPE, CAGR (2021-2028)
FIGURE 28 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY TRANSPLANT TYPE, LIFELINE CURVE
FIGURE 29 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY APPLICATION, 2020
FIGURE 30 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY APPLICATION, 2021-2028 (USD MILLION)
FIGURE 31 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 32 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY END USERS, 2021-2028 (USD MILLION)
FIGURE 34 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY END USERS, CAGR (2021-2028)
FIGURE 35 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: END USERS, CAGR (2021-2028)
FIGURE 36 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY END USERS, LIFELINE CURVE
FIGURE 37 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: SNAPSHOT (2020)
FIGURE 38 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY COUNTRY (2020)
FIGURE 39 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY COUNTRY (2021 & 2028)
FIGURE 40 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY COUNTRY (2020 & 2028)
FIGURE 41 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: BY PRODUCT & SERVICES (2021-2028)
FIGURE 42 EUROPE HUMAN LEUKOCYTE ANTIGEN (HLA) TYPING FOR TRANSPLANT MARKET: COMPANY SHARE 2020 (%)
FIGURE 43 NORTH AMERICA SEQUENCING BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 44 EUROPE SEQUENCING BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 45 ASIA-PACIFIC SEQUENCING BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 46 SOUTH AMERICA SEQUENCING BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 47 MIDDLE EAST AND AFRICA SEQUENCING BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 48 NORTH AMERICA NGS SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 49 EUROPE NGS SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 50 ASIA-PACIFIC NGS SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 51 SOUTH AMERICA NGS SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 52 MIDDLE EAST AND AFRICA NGS SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 53 NORTH AMERICA SANGER SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 54 EUROPE SANGER SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 55 ASIA-PACIFIC SANGER SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 56 SOUTH AMERICA SANGER SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 57 MIDDLE EAST AND AFRICA SANGER SEQUENCING: COMPANY SHARE 2020 (%)
FIGURE 58 NORTH AMERICA PCR BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 59 EUROPE PCR BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 60 ASIA-PACIFIC PCR BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 61 SOUTH AMERICA PCR BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 62 MIDDLE EAST AND AFRICA PCR BASED MOLECULAR ASSAYS: COMPANY SHARE 2020 (%)
FIGURE 63 NORTH AMERICA REAL TIME PCR: COMPANY SHARE 2020 (%)
FIGURE 64 EUROPE REAL TIME PCR: COMPANY SHARE 2020 (%)
FIGURE 65 ASIA-PACIFIC REAL TIME PCR: COMPANY SHARE 2020 (%)
FIGURE 66 SOUTH AMERICA REAL TIME PCR: COMPANY SHARE 2020 (%)
FIGURE 67 MIDDLE EAST AND AFRICA REAL TIME PCR: COMPANY SHARE 2020 (%)
FIGURE 68 NORTH AMERICA SEQUENCE-SPECIFIC PRIMER-PCR: COMPANY SHARE 2020 (%)
FIGURE 69 EUROPE SEQUENCE-SPECIFIC PRIMER-PCR: COMPANY SHARE 2020 (%)
FIGURE 70 ASIA-PACIFIC SEQUENCE-SPECIFIC PRIMER-PCR: COMPANY SHARE 2020 (%)
FIGURE 71 SOUTH AMERICA SEQUENCE-SPECIFIC PRIMER-PCR: COMPANY SHARE 2020 (%)
FIGURE 72 MIDDLE EAST AND AFRICA SEQUENCE-SPECIFIC PRIMER-PCR: COMPANY SHARE 2020 (%)
FIGURE 73 NORTH AMERICA SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR: COMPANY SHARE 2020 (%)
FIGURE 74 EUROPE SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR: COMPANY SHARE 2020 (%)
FIGURE 75 ASIA-PACIFIC SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR: COMPANY SHARE 2020 (%)
FIGURE 76 SOUTH AMERICA SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR: COMPANY SHARE 2020 (%)
FIGURE 77 MIDDLE EAST AND AFRICA SEQUENCE-SPECIFIC OLIGONUCLEOTIDE-PCR: COMPANY SHARE 2020 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.














