Europe Acute Myeloid Leukemia Diagnostics Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
702.84 Million
USD
1,662.45 Million
2022
2030
USD
702.84 Million
USD
1,662.45 Million
2022
2030
| 2023 –2030 | |
| USD 702.84 Million | |
| USD 1,662.45 Million | |
|
|
|
|
Mercado de diagnóstico de leucemia mieloide aguda na Europa, por tipo de produto (instrumentos e consumíveis e acessórios), tipo de teste (teste de imagem, teste de sangue, testes de medula óssea , teste de biomarcador, imunofenotipagem, testes genéticos e outros) , tipo de cancro (mieloblástico (M0) , Mieloblástico (M1), Mieloblástico (M2), Promielocítico (M3), Mielomonocítico (M4), Monocítico (M5), Eritroleucemia (M6) e Megacariocítico (M7)), Faixa etária (abaixo dos 21, 21-29, 30- 65 e 65 e acima), género (masculino e feminino), utilizador final (hospital, laboratórios associados, laboratórios de diagnóstico independentes, centros de diagnóstico por imagem, institutos de investigação do cancro e outros) , canal de distribuição (licitação direta e vendas a retalho) Tendências do setor e Previsão para 2030.
Análise e Insights do Mercado de Diagnóstico da Leucemia Mieloide Aguda na Europa
Devido ao aumento dos gastos em saúde, existe um impulso no crescimento do mercado, o que pode resultar em melhores provisões de oportunidades de I&D. Além disso, o aumento da investigação clínica e dos laboratórios de diagnóstico para o diagnóstico do cancro estão a permitir a expansão do mercado.
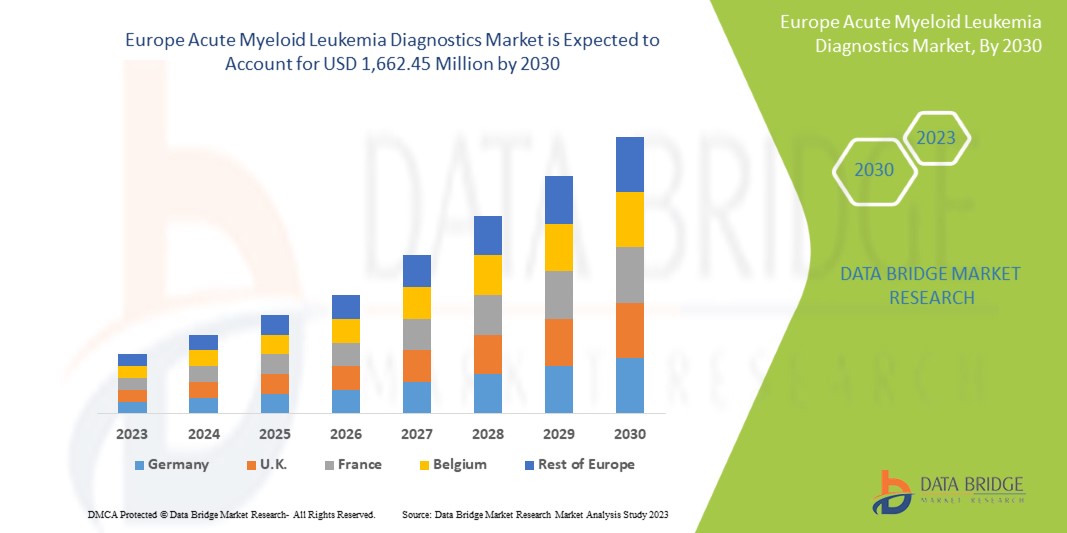
O mercado europeu de diagnóstico de leucemia mieloide aguda deverá crescer no período previsto de 2023 a 2030. A Data Bridge Market Research analisa que o mercado está a crescer com um CAGR de 11,4% no período previsto de 2023 a 2030 e deverá atingir os 1.662,45 milhões de USD até 2030, face aos 702,84 milhões de USD em 2022.
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2023 a 2030 |
|
Ano base |
2022 |
|
Ano Histórico |
2021 (Personalizável para 2020-2016) |
|
Unidades quantitativas |
Receita em milhões de dólares americanos |
|
Segmentos abrangidos |
Por tipo de produto (instrumentos e consumíveis e acessórios), tipo de teste (teste de imagem, teste sanguíneo, testes de medula óssea, teste de biomarcador, imunofenotipagem, testes genéticos e outros), tipo de cancro (mieloblástico (M0), mieloblástico (M1), mieloblástico (M2), Promielocítico (M3), Mielomonocítico (M4), Monocítico (M5), Eritroleucemia (M6) e Megacariocítico (M7)), Faixa etária (abaixo dos 21, 21-29, 30-65 e 65 e acima) , Género (masculino e feminino), Utilizador final (hospital, laboratórios associados, laboratórios de diagnóstico independentes, centros de diagnóstico por imagem, institutos de investigação do cancro e outros), Canal de distribuição (licitação direta e vendas no retalho). |
|
Países abrangidos |
Alemanha, Reino Unido, França, Itália, Espanha, Rússia, Países Baixos, Suíça, Bélgica, Turquia e resto da Europa. |
|
Atores do mercado abrangidos |
Epigenomics AG, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, BIOMERIEUX, Merck KGaA, DiaSorin SpA, Koninklijke Philips NV, Sonic Healthcare e SternMed GmbH, entre outras. |
Definição de Mercado
A forma mais comum de cancro do sangue, a leucemia mieloide aguda (LMA), é também uma das formas pouco frequentes de leucemia. Este tipo de cancro invade o sangue e depois espalha-se para órgãos e sistemas corporais próximos. Os especialistas devem diagnosticar manualmente as células cancerígenas e não cancerígenas, examinando as imagens das células ao microscópio e fornecendo etiquetas através de anotações.
O aumento dos gastos com a saúde pode resultar em melhores provisões de oportunidades de I&D, o que também impulsiona o crescimento do negócio. A crescente quantidade de laboratórios de investigação clínica e de diagnóstico para pré-testes de cancro está a permitir que o mercado se expanda.
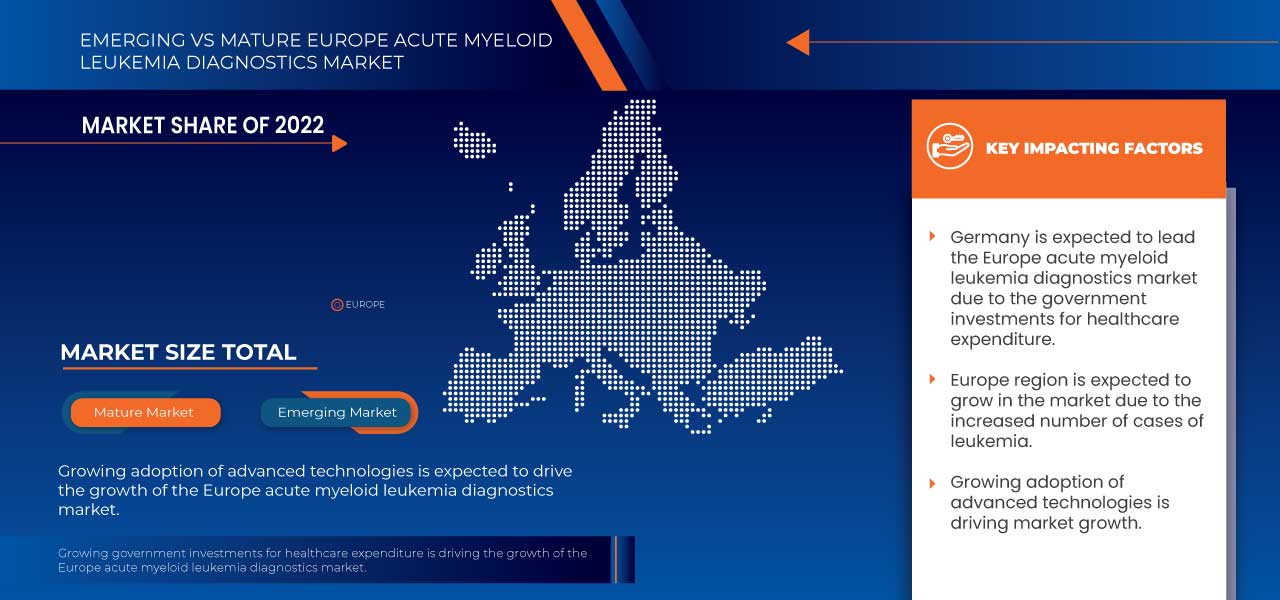
O número crescente de casos de cancro e a consciencialização sobre a leucemia levaram ao crescimento do mercado.
O mercado europeu de diagnóstico da leucemia mieloide aguda está a crescer no ano previsto devido ao aumento de participantes no mercado e à disponibilidade de instrumentos de imagem e consumíveis avançados. Juntamente com isto, os fabricantes estão envolvidos em atividades de I&D para lançar produtos no mercado. O aumento da I&D e dos pré-testes estão a impulsionar ainda mais o crescimento do mercado. No entanto, espera-se que as regulamentações e normas rigorosas para a aprovação e comercialização de produtos de diagnóstico de cancro e de diagnóstico tardio representem um desafio ao crescimento do mercado.
Dinâmica do mercado de diagnósticos de leucemia mieloide aguda na Europa
Esta secção trata da compreensão dos impulsionadores, restrições, oportunidades e desafios do mercado. Tudo isto é discutido em detalhe abaixo:
MOTORISTAS
- Prevalência crescente de cancro de leucemia
Todas as idades podem ser afetadas pela leucemia. A leucemia pode ser difícil de diagnosticar porque, apesar da sua vasta gama de sinais e sintomas, não é específica e pode estar relacionada com outras condições médicas mais comuns. A LMA é um dos quatro tipos comuns de leucemia em adultos. Ocorre com menos frequência. É um pouco mais comum entre homens do que mulheres, no entanto, o risco médio ao longo da vida de desenvolver LMA em ambos os sexos é de cerca de ½ de 1% em média.
A LMA é o segundo tipo mais comum de leucemia diagnosticada em adultos e crianças, mas a maioria dos casos ocorre em adultos. Embora possa ser diagnosticado em qualquer idade, é pouco frequente antes dos 45 anos.
Devido a vários fatores de risco, a incidência de LMA tem vindo a aumentar, tornando-se um problema socioeconómico significativo. Espera-se que isto atue como um impulsionador do crescimento do mercado.
- Novos avanços tecnológicos no diagnóstico da leucemia
A forma mais comum de cancro do sangue, a LMA, é também uma das formas pouco frequentes de leucemia. Este tipo de cancro invade o sangue e depois espalha-se para órgãos e sistemas corporais próximos. Os especialistas devem diagnosticar manualmente as células cancerígenas e não cancerígenas, examinando as imagens das células ao microscópio e fornecendo etiquetas através de anotações. No entanto, este exame microscópico da mão consome tempo e pode dar um diagnóstico incorreto.
O risco de prescrever medicamentos incorretos foi então reduzido através de software computorizado. A criação de um sistema de classificação automático e fiável tornou-se vital para travar os efeitos devastadores da doença da leucemia. As técnicas de segmentação múltipla constituíram a base dos algoritmos de classificação de leucemias existentes.
O desenvolvimento de vários novos métodos de diagnóstico aumentará o crescimento do mercado à medida que muitos produtos novos e avançados forem lançados. Assim, espera-se que haja procura no mercado por produtos de diagnóstico de cancro leucémico agudo.
RESTRIÇÕES
- Regulamentação e normas rigorosas para aprovação e comercialização de produtos de diagnóstico de leucemia
As regulamentações rigorosas para a comercialização de qualquer produto no mercado estão a revelar-se um grande desafio para os fabricantes de produtos de diagnóstico do cancro que têm regulamentos e um organismo diferente para os procedimentos regulamentares.
Os fabricantes precisam primeiro de verificar a aprovação da marca CE para a comercialização dos seus produtos no mercado. Espera-se que as políticas regulamentares rigorosas dificultem o crescimento e o desenvolvimento do mercado.
- Diagnóstico tardio e mau prognóstico da leucemia
O cancro é a principal causa de morte no mundo. No entanto, se forem identificados precocemente e receberem os cuidados adequados, alguns tipos de cancro podem ser curados. O processo de diagnóstico pode sofrer atrasos no diagnóstico do cancro. Quando os doentes ignoram ou não respondem a possíveis sinais de cancro, o diagnóstico é adiado. A principal causa da apresentação tardia é a falta de conhecimento público sobre os sinais precoces do cancro, especialmente se estes sintomas forem pouco frequentes.
O diagnóstico tardio decorre muitas vezes destes fatores, o que resulta num mau prognóstico. Portanto, espera-se que isto restrinja o crescimento do mercado.
OPORTUNIDADE/DESAFIOS
- Aumento de custos, segurança e questões de conveniência
A leucemia é um cancro fatal, e o processo de diagnóstico da leucemia também apresenta problemas de segurança. Não é rentável. Um dos distúrbios médicos mais dispendiosos de tratar é o cancro. Os doentes oncológicos podem ser hospitalizados e receber uma variedade de terapias, como cirurgia, radioterapia e terapia sistémica. Os prémios de seguro de saúde para doentes oncológicos são agora mais caros do que no passado. Além disso, os seus custos de copagamento, franquia e cosseguro estão a aumentar.
Doravante, o atual processo de diagnóstico da leucemia apresenta problemas de segurança, custo e conveniência, o que deverá representar um desafio ao crescimento do mercado.
Desenvolvimentos recentes
- Em novembro de 2022, a Canon Inc. adquiriu a Redlen Technologies Inc. (Redlen), uma das empresas líderes mundiais na criação de novas tecnologias relacionadas com o desenvolvimento e fabrico de módulos detetores de semicondutores. A Canon Medical Systems Corporation (Canon Medical), uma empresa do grupo Canon Inc., desenvolveu o primeiro sistema de TC de contagem de fotões (PCCT) produzido internamente, incorporando as tecnologias avançadas da Redlen. Este sistema foi instalado no Centro Nacional de Cancro (NCC) de Investigação Oncológica Exploratória e Centro de Ensaios Clínicos no Japão, onde é atualmente utilizado para conduzir investigação que explora as aplicações clínicas da PCCT.
- Em outubro de 2022, o presidente da Sysmex Corporation e o seu CEO: Hisashi Ietsugu anunciam a aprovação de um pedido de alteração parcial na aprovação de fabrico e comercialização no Japão do seu reagente de amplificação genética LYNOAMP CK19 comercializado como reagente de teste de metástase de linfonodo para o cancro da mama , cancro colorretal, cancro gástrico e cancro do pulmão de células não pequenas, expandindo a sua amplificação para o cancro do colo do útero e cancro do endométrio.
Âmbito do mercado de diagnósticos de leucemia mieloide aguda na Europa
O mercado europeu de diagnóstico da leucemia mieloide aguda está segmentado em sete segmentos notáveis com base no tipo de produto, tipo de teste, tipo de cancro, faixa etária, género, utilizador final e canal de distribuição. O crescimento entre estes segmentos irá ajudá-lo a analisar os principais segmentos de crescimento nos setores e fornecerá aos utilizadores uma visão geral valiosa do mercado e informações de mercado para tomar decisões estratégicas para identificar as principais aplicações de mercado.
Tipo de produto
- Instrumentos
- Consumíveis e Acessórios
Com base no tipo de produto, o mercado está segmentado em instrumentos, consumíveis e acessórios.
Tipo de teste
- Exame de sangue
- Teste de imagem
- Teste de Medula Óssea
- Testes genéticos
- Teste de biomarcador
- Imunofenotipagem
- Outros
Com base no tipo de teste, o mercado está segmentado em análises ao sangue, exames de imagem, exames de medula óssea, testes genéticos, testes de biomarcadores, imunofenotipagem e outros.
Tipo de cancro
- Mieloblástico (M0)
- Mieloblástico (M1)
- Mieloblástico (M2)
- Promielocítico (M3)
- Mielomonocítico (M4)
- Monocítico (M5)
- Eritroleucemia (M6)
- Megacariocítico (M7)
Com base no tipo de cancro, o mercado está segmentado em mieloblástico (M0), mieloblástico (M1), mieloblástico (M2), promielocítico (M3), mielomonocítico (M4), monocítico (M5), eritroleucémico (M6) e megacariocítico (M7 ) .
Faixa etária
- Abaixo de 21
- 21-29
- 30-65
- 65 e acima
Com base na faixa etária, o mercado está segmentado em menores de 21, 21 a 29, 30 a 65 e 65 anos ou mais.
Género
- Macho
- Fêmea
Com base no género, o mercado está segmentado em masculino e feminino.
Utilizador final
- Hospital
- Laboratórios Associados
- Laboratórios de Diagnóstico Independentes
- Centros de Diagnóstico por Imagem
- Institutos de Investigação do Cancro
- Outros
Com base no utilizador final, o mercado está segmentado em hospitais, laboratórios associados, laboratórios de diagnóstico independentes, centros de diagnóstico por imagem, institutos de investigação do cancro e outros.
Canal de Distribuição
- Licitação Direta
- Vendas no retalho
Com base no canal de distribuição, o mercado está segmentado em licitação direta e vendas a retalho.
Análise/Insights Regionais do Mercado de Diagnóstico Aguda da Leucemia Mieloide na Europa
O mercado europeu de diagnóstico de leucemia mieloide aguda é analisado e são fornecidos insights e tendências sobre o tamanho do mercado por país com base no tipo de produto, tipo de teste, tipo de cancro, faixa etária, género, utilizador final e canal de distribuição .
Os países abrangidos neste relatório de mercado são a Alemanha, o Reino Unido, a França, a Itália, a Espanha, a Rússia, os Países Baixos, a Suíça, a Bélgica, a Turquia e o resto da Europa, conforme referenciado acima.
A Alemanha domina o mercado europeu de diagnóstico de leucemia mieloide aguda em termos de quota de mercado e receitas e continuará a aumentar o seu domínio durante o período previsto. Isto deve-se aos crescentes avanços tecnológicos em instrumentos de diagnóstico e consumíveis na região, aos crescentes investimentos em I&D e ao lançamento de novos produtos que estão a impulsionar o crescimento do mercado.
A secção de países do relatório também fornece fatores individuais que impactam o mercado e alterações nas regulamentações do mercado que impactam as tendências atuais e futuras do mercado. Pontos de dados, como vendas de produtos novos e de reposição, demografia do país, epidemiologia de doenças e tarifas de importação e exportação são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e a disponibilidade das marcas europeias e os desafios enfrentados devido à concorrência das marcas locais e nacionais e o impacto dos canais de vendas são considerados ao fornecer uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado dos diagnósticos de leucemia mieloide aguda na Europa
O panorama competitivo do mercado europeu de diagnóstico de leucemia mieloide aguda fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em I&D, novas iniciativas de mercado, presença na Europa, localizações e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento do produto, amplitude e amplitude do produto e domínio da aplicação . Os pontos de dados fornecidos acima estão apenas relacionados com o foco das empresas em relação ao mercado.
Alguns dos principais participantes que operam no mercado europeu de diagnóstico de leucemia mieloide aguda são a Epigenomics AG, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, BIOMERIEUX, Merck KGaA, DiaSorin SpA, Koninklijke Philips NV, Sonic Healthcare e SternMed GmbH, entre outros.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, REGULATIONS
5.1 REGULATORY SCENARIO IN THE U.S.
5.2 REGULATORY SCENARIO IN AUSTRALIA
5.3 REGULATORY SCENARIO IN JAPAN
5.4 REGULATORY SCENARIO IN CHINA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING PREVALENCE OF LEUKEMIA CANCER
6.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN LEUKEMIA DIAGNOSTICS
6.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
6.1.4 INCREASE IN AWARENESS REGARDING LEUKEMIA CANCER
6.2 RESTRAINTS
6.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF LEUKEMIA DIAGNOSTIC PRODUCTS
6.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF LEUKEMIA
6.3 OPPORTUNITIES
6.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR LEUKEMIA
6.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
6.3.3 GOVERNMENT INITIATIVES TOWARD CANCER DIAGNOSTICS
6.4 CHALLENGES
6.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
6.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 INSTRUMENTS
7.2.1 BIOPSY INSTRUMENTS
7.2.1.1 BONE MARROW BIOPSY
7.2.1.2 NEEDLE BIOPSY
7.2.1.3 SURGICAL BIOPSY
7.2.1.4 OTHERS
7.2.2 PATHOLOGY-BASED INSTRUMENTS
7.2.2.1 PCR INSTRUMENTS
7.2.2.2 SLIDE STAINING SYSTEMS
7.2.2.3 TISSUE PROCESSING SYSTEMS
7.2.2.4 CELL PROCESSORS
7.2.2.5 OTHER PATHOLOGY-BASED INSTRUMENTS
7.2.3 IMAGING INSTRUMENTS
7.2.3.1 ULTRASOUND SYSTEMS
7.2.3.2 CT SYSTEMS
7.2.3.3 MRI SYSTEMS
7.2.3.4 OTHERS
7.2.4 OTHERS
7.3 CONSUMABLES & ACCESSORIES
7.3.1 KITS
7.3.1.1 PCR KITS
7.3.1.2 DNA POLYMERASE KITS
7.3.1.3 NUCLEIC ACID ISOLATION KITS
7.3.1.4 OTHERS
7.3.2 REAGENTS
7.3.2.1 ASSAYS
7.3.2.2 BUFFERS
7.3.2.3 PRIMERS
7.3.2.4 OTHERS
7.3.3 PROBES
7.3.4 OTHER CONSUMABLES
8 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 BLOOD TEST
8.2.1 COMPLETE BLOOD COUNT (CBC)
8.2.2 BLOOD CHEMISTRY TESTS
8.2.3 OTHERS
8.3 IMAGING TEST
8.3.1 COMPUTED TOMOGRAPHY (CT) SCAN
8.3.2 MRI
8.3.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
8.3.4 OTHERS
8.4 BONE MARROW TESTS
8.4.1 BONE MARROW ASPIRATE
8.4.2 BONE MARROW BIOPSY
8.4.3 OTHERS
8.5 GENETIC TESTS
8.5.1 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
8.5.2 KARYOTYPING
8.5.3 OTHERS
8.6 BIOMARKER TEST
8.6.1 GENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.2 EPIGENETIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.6.3 PROTEOMIC ACUTE MYELOID LEUKEMIA (AML) BIOMARKER
8.7 IMMUNOPHENOTYPING
8.7.1 FLOW CYTOMETRY
8.7.2 IMMUNOHISTOCHEMISTRY
8.7.3 OTHERS
8.8 OTHERS
9 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE
9.1 OVERVIEW
9.2 MYELOBLASTIC (M0)
9.3 MYELOBLASTIC (M1)
9.4 MYELOBLASTIC (M2)
9.5 PROMYELOCYTIC (M3)
9.6 MYELOMONOCYTIC (M4)
9.7 MONOCYTIC (M5)
9.8 ERYTHROLEUKEMIA (M6)
9.9 MEGAKARYOCYTIC (M7)
10 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP
10.1 OVERVIEW
10.2 65 AND ABOVE
10.3 30-65
10.4 BELOW 21
10.5 21-29
11 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER
11.1 OVERVIEW
11.2 MALE
11.3 FEMALE
12 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 ASSOCIATED LABS
12.4 INDEPENDENT DIAGNOSTIC LABORATORIES
12.5 DIAGNOSTIC IMAGING CENTERS
12.6 CANCER RESEARCH INSTITUTES
12.7 OTHERS
13 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 UNITED KINGDOM
14.1.3 FRANCE
14.1.4 ITALY
14.1.5 SPAIN
14.1.6 RUSSIA
14.1.7 NETHERLANDS
14.1.8 SWITZERLAND
14.1.9 BELGIUM
14.1.10 TURKEY
14.1.11 REST OF EUROPE
15 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 CANON MEDICAL SYSTEMS CORPORATION.
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 SYSMEX CORPORATION
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 EPIGENOMICS AG.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 MYRIAD GENETICS, INC..
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 F. HOFFMANN- LA ROCHE LTD
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 ABBOTT
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 AGILENT TECHNOLOGIES, INC.
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 BD
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENT
17.9 BIOMERIEUX
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.1 BIO-RAD LABORATORIES, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 DIASORIN S.P.A.
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 EXACT SCIENCES CORPORATION
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 FONAR CORP.
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 HOLOGIC INC.
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENT
17.15 ILLUMINA, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
17.16 KONINKLIJKE PHILIPS N.V.
17.16.1 COMPANY SNAPSHOT
17.16.2 REVENUE ANALYSIS
17.16.3 PRODUCT PORTFOLIO
17.16.4 RECENT DEVELOPMENT
17.17 MEDONICA CO. LTD
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 MERCK KGAA
17.18.1 COMPANY SNAPSHOT
17.18.2 REVENUE ANALYSIS
17.18.3 PRODUCT PORTFOLIO
17.18.4 RECENT DEVELOPMENTS
17.19 MINFOUND MEDICAL SYSTEMS CO., LTD
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 PLEXBIO
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENTS
17.21 QIAGEN
17.21.1 COMPANY SNAPSHOT
17.21.2 REVENUE ANALYSIS
17.21.3 PRODUCT PORTFOLIO
17.21.4 RECENT DEVELOPMENTS
17.22 QUEST DIAGNOSTICS INCORPORATED
17.22.1 COMPANY SNAPSHOT
17.22.2 REVENUE ANALYSIS
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENTS
17.23 SIEMENS HEALTHCARE GMBH
17.23.1 COMPANY SNAPSHOT
17.23.2 REVENUE ANALYSIS
17.23.3 PRODUCT PORTFOLIO
17.23.4 RECENT DEVELOPMENT
17.24 SONIC HEALTHCARE
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
17.25 STERNMED GMBH
17.25.1 COMPANY SNAPSHOT
17.25.2 PRODUCT PORTFOLIO
17.25.3 RECENT DEVELOPMENTS
17.26 THERMO FISHER SCIENTIFIC INC.
17.26.1 COMPANY SNAPSHOT
17.26.2 REVENUE ANALYSIS
17.26.3 PRODUCT PORTFOLIO
17.26.4 RECENT DEVELOPMENT
17.27 TIME MEDICAL HOLDING
17.27.1 COMPANY SNAPSHOT
17.27.2 PRODUCT PORTFOLIO
17.27.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tabela
TABLE 1 APPROVED DIAGNOSTICS OF LEUKEMIA
TABLE 2 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 EUROPE INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 EUROPE INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 EUROPE PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 EUROPE IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 8 EUROPE CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 EUROPE CONSUMABLES AND ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 EUROPE KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 EUROPE REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 12 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 EUROPE BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 15 EUROPE IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 EUROPE IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 17 EUROPE BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 EUROPE BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 19 EUROPE GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 EUROPE GENETIC TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 EUROPE BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 EUROPE IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 EUROPE IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 27 EUROPE MYELOBLASTIC (M0) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 EUROPE MYELOBLASTIC (M1) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 EUROPE MYELOBLASTIC (M2) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 EUROPE PROMYELOCYTIC (M3) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 EUROPE MYELOMONOCYTIC (M4) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 EUROPE MONOCYTIC (M5) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 EUROPE ERYTHROLEUKEMIA (M6) IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 EUROPE MEGAKARYOCYTIC (M7) ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 36 EUROPE 65 AND ABOVE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 EUROPE 30-65 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 EUROPE BELOW 21 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 EUROPE 21-29 IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 41 EUROPE MALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 EUROPE FEMALE IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 44 EUROPE HOSPITALS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 EUROPE ASSOCIATED LABS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 EUROPE INDEPENDENT DIAGNOSTIC LABORATORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 EUROPE DIAGNOSTIC IMAGING CENTERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 EUROPE CANCER RESEARCH INSTITUTES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 EUROPE OTHERS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 EUROPE DIRECT TENDER IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 EUROPE RETAIL SALES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 54 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 55 EUROPE INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 EUROPE PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 57 EUROPE IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 58 EUROPE BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 59 EUROPE CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 EUROPE KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 EUROPE REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 63 EUROPE IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 EUROPE BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 65 EUROPE BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 66 EUROPE BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 EUROPE IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 EUROPE GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 70 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 71 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 72 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 73 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 74 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 GERMANY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 GERMANY PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 77 GERMANY IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 78 GERMANY BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 GERMANY CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 GERMANY KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 GERMANY REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 83 GERMANY IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 84 GERMANY BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 85 GERMANY BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 86 GERMANY BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 GERMANY IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 GERMANY GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 90 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 91 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 92 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 93 GERMANY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 94 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 UNITED KINGDOM INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 UNITED KINGDOM PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 UNITED KINGDOM IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 UNITED KINGDOM BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 UNITED KINGDOM CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 UNITED KINGDOM KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 UNITED KINGDOM REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 UNITED KINGDOM IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 UNITED KINGDOM BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 UNITED KINGDOM BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 UNITED KINGDOM BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 UNITED KINGDOM IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 UNITED KINGDOM IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 110 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 111 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 112 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 UNITED KINGDOM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 FRANCE INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 FRANCE PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 FRANCE IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 FRANCE BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 FRANCE CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 FRANCE KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 FRANCE REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 123 FRANCE IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 124 FRANCE BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 125 FRANCE BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 126 FRANCE BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 127 FRANCE IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 FRANCE GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 130 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 131 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 132 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 133 FRANCE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 134 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 135 ITALY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 136 ITALY PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 137 ITALY IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 138 ITALY BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 139 ITALY CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 140 ITALY KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 141 ITALY REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 142 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 143 ITALY IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 144 ITALY BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 145 ITALY BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 146 ITALY BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 147 ITALY IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 148 ITALY GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 149 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 150 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 151 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 152 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 153 ITALY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 154 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 155 SPAIN INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 156 SPAIN PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 157 SPAIN IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 158 SPAIN BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 159 SPAIN CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 160 SPAIN KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 161 SPAIN REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 162 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 SPAIN IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 SPAIN BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 165 SPAIN BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 SPAIN BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 SPAIN IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 168 SPAIN GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 169 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 170 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 171 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 172 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 173 SPAIN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 174 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 175 RUSSIA INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 176 RUSSIA PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 177 RUSSIA IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 178 RUSSIA BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 179 RUSSIA CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 180 RUSSIA KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 181 RUSSIA REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 182 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 183 RUSSIA IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 184 RUSSIA BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 185 RUSSIA BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 186 RUSSIA BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 187 RUSSIA IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 188 RUSSIA GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 189 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 190 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 191 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 192 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 RUSSIA ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 194 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 195 NETHERLANDS INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 196 NETHERLANDS PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 197 NETHERLANDS IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 198 NETHERLANDS BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 199 NETHERLANDS CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 200 NETHERLANDS KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 201 NETHERLANDS REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 202 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 203 NETHERLANDS IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 204 NETHERLANDS BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 205 NETHERLANDS BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 206 NETHERLANDS BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 207 NETHERLANDS IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 208 NETHERLANDS GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 209 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 210 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 211 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 212 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 214 NETHERLANDS ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 215 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 216 SWITZERLAND INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 217 SWITZERLAND PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 218 SWITZERLAND IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 219 SWITZERLAND BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 220 SWITZERLAND CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 221 SWITZERLAND KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 222 SWITZERLAND REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 223 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 224 SWITZERLAND IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 225 SWITZERLAND BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 226 SWITZERLAND BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 227 SWITZERLAND BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 228 SWITZERLAND IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 229 SWITZERLAND GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 230 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 231 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 232 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 233 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 234 SWITZERLAND ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 235 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 236 BELGIUM INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 237 BELGIUM PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 238 BELGIUM IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 239 BELGIUM BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 240 BELGIUM CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 241 BELGIUM KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 242 BELGIUM REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 243 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 244 BELGIUM IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 245 BELGIUM BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 246 BELGIUM BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 247 BELGIUM BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 248 BELGIUM IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 249 BELGIUM GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 250 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 251 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 252 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 253 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 254 BELGIUM ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 255 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 256 TURKEY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 257 TURKEY PATHOLOGY-BASED INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 258 TURKEY IMAGING INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 259 TURKEY BIOPSY INSTRUMENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 260 TURKEY CONSUMABLES & ACCESSORIES IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 261 TURKEY KITS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 262 TURKEY REAGENTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 263 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 264 TURKEY IMAGING TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 265 TURKEY BONE MARROW TESTS IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 266 TURKEY BLOOD TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 267 TURKEY BIOMARKER TEST IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 268 TURKEY IMMUNOPHENOTYPING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 269 TURKEY GENETIC TESTING IN ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 270 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 271 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 272 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY GENDER, 2021-2030 (USD MILLION)
TABLE 273 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 274 TURKEY ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 275 REST OF EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
Lista de Figura
FIGURE 1 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE GROWING PREVALENCE OF LEUKEMIA CANCER IS EXPECTED TO DRIVE THE EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INSTRUMENTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET
FIGURE 14 NUMBER OF PREVALENCE OF LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 15 FIVE YEARS PREVALENCE LEUKEMIA INCIDENCE WORLDWIDE (BOTH SEXES)
FIGURE 16 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 17 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 18 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 19 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 21 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 22 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 23 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 24 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 25 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 26 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 27 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 28 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 29 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 30 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 31 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 32 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2022
FIGURE 33 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, 2023-2030 (USD MILLION)
FIGURE 34 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, CAGR (2023-2030)
FIGURE 35 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 36 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 37 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 38 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 39 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 41 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 42 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 43 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 45 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 46 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 47 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 48 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: PRODUCT TYPE (2023-2030)
FIGURE 49 EUROPE ACUTE MYELOID LEUKEMIA DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.