Asia Pacific Active Medical Implantable Devices Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
5.06 Billion
USD
11.83 Billion
2025
2033
USD
5.06 Billion
USD
11.83 Billion
2025
2033
| 2026 –2033 | |
| USD 5.06 Billion | |
| USD 11.83 Billion | |
|
|
|
|
Asia-Pacific Active Medical Implantable Devices Market, By Product (Cardiac Resynchronization Therapy Devices (CRT-D), Implantable Cardioverter Defibrillators, Implantable Cardiac Pacemakers, Eye Implants, Neurostimulators, Active Implantable Hearing Devices, Ventricular Assist Devices, Implantable Heart Monitors/Insertable Loop recorders, Brachytheraphy, Implantable Glucose Monitors, Dropped Foot Implants, Shoulder Implants, Implantable Infusion Pumps, and Implantable Accessories), Surgery Type (Traditional Surgical Methods and Minimally Invasive Surgery), Procedure (Neurovascular, Cardiovascular, Hearing, and Others), End User (Hospitals, Specialty Clinics, Ambulatory Surgical Centers, Clinics), Country (Japan, China , Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific) Industry Trends and Forecast to 2028
Market Analysis and Insights: Asia-Pacific Active Medical Implantable Devices Market
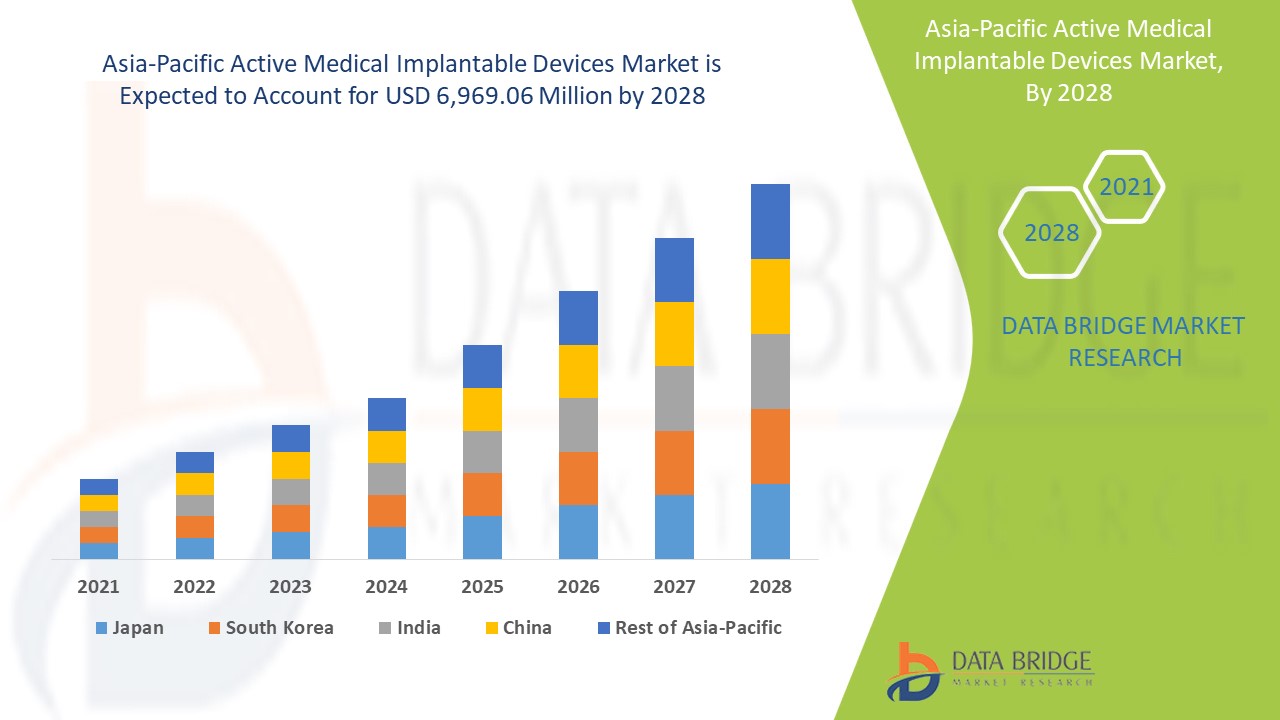
Asia-Pacific active medical implantable devices market is expected to gain significant growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyzes that the market is growing with a CAGR of 11.2% in the forecast period of 2021 to 2028 and is expected to reach USD 6,969.06 million by 2028. Rise in chronic diseases such as cardiovascular are the major drivers propelling the growth of the market in the forecast period. However the high cost of treatment with implantable devices is retaining the market growth. Increase in healthcare expenditure is providing an opportunity for the market to grow. On the other hand, decrease in the number of professional workers is challenging the market for implantable devices.
Active medical implantable device are the devices which rely on an outer power source instead of the body or gravity and these devices are designed to be introduced into the body with the intention to remain there for the following procedure or treatment.
For Active medical implantable devices market the growth in R & D and the advancement in the treatment and products will impact the manufacturer in launching new product into the market which will enhance its the market growth. Currently various research studies are taking place which is expected to create a competitive advantage for manufacturers to develop new and innovative active medical implantable devices which is expected to provide various other opportunities in the active medical implantable devices market. However, the expensive treatment and other hospitalization cost is expected to restraint the market growth in the forecast period.
The Asia-Pacific active medical implantable devices market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an analyst brief, our team will help you create a revenue impact solution to achieve your desired goal.
Asia-Pacific Active Medical Implantable Devices Market Scope and Market Size
Asia-Pacific active medical implantable devices market is categorized into four notable segments which are based on the product, surgery type, procedure, end users.
- On the basis of product, the Asia-Pacific active medical implantable devices market is segmented into Cardiac Resynchronization Therapy Devices (CRT-D), implantable cardioverter defibrillators, implantable cardiac pacemakers, eye implants, neurostimulators, active implantable hearing devices, ventricular assist devices, implantable heart monitors/insertable loop recorders, brachytheraphy, implantable glucose monitors, dropped foot implants, shoulder implants, implantable infusion pumps and implantable accessories. In 2021, Cardiac Resynchronization Therapy Devices (CRT-D) segment is expected to dominate the market owing to increase in the prevalence of cardiovascular diseases Asia-Pacific.
- On the basis of surgery type the Asia-Pacific active medical implantable devices market is segmented into traditional surgical methods and minimally invasive surgery. In 2021, traditional surgical methods segment is expected to dominate the market as it has reasonable cost and expense of the treatment.
- On the basis of procedure, the Asia-Pacific active medical implantable devices market is segmented into neurovascular, cardiovascular, hearing, and others. In 2021, cardiovascular is expected to dominate the market with the increase in cardiovascular diseases.
- On the basis of end-users, the Asia-Pacific active medical implantable devices market is segmented into hospitals, specialty clinics, ambulatory surgical centers, and clinics. In 2021, hospital segment is expected to dominate the market due growing demand for active medical implantable devices.
Active Medical Implantable Devices Market Country Level Analysis
The active medical implantable devices market is analyzed and market size information is provided on the basis of product, surgery type, procedure, and end users.
The countries covered in the active medical implantable devices market report are the Japan, China , Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific.
Active medical implantable devices segment in Asia-Pacific region is expected to grow with the highest growth rate in the forecast period of 2021 to 2028 because of increasing number of active medical implantable device manufacturers. China is leading the growth of the Asia-Pacific active medical implantable devices market and hospital segment is dominating in this country due to increasing number of fire incidence in the country.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Increase in FDA approval are boosting the market growth of active medical implantable devices
Active medical implantable devices market also provides you with detailed market analysis for every country growth in Active medical implantable devices industry with Active medical implantable devices drugs sales, impact of advancement in the Active medical implantable devices technology and changes in regulatory scenarios with their support for the market. The data is available for historic period 2010 to 2018.
Competitive Landscape and Active Medical Implantable Devices Market Share Analysis
Active medical implantable devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to Active medical implantable devices market.
The major companies which are dealing in the Asia-Pacific active medical implantable devices market are NeuroPace, Inc., Axonics, Inc., Stimwave LLC, NEVRO CORP, Second Sight, BIOTRONIK, ABIOMED, Boston Scientific Corporation, Medtronic, Abbott, Eckert & Ziegler., Sonova, Zhejiang Nurotron Biotechnology Co., Ltd, Demant A/S, Cochlear Ltd, Microson, Oticon Medical, Nano Retina, GluSense, MED-EL Medical Electronics among others.
Several product launches and agreements are also initiated by the companies worldwide, which are also accelerating the growth of the active medical implantable devices market.
For instance,
- In January 2021, Boston Scientific announced that it has entered into an agreement to acquire Preventice Solutions, Inc. The acquisition will help the company to expand its cardiac portfolio.
- In July 2021, Abbott announced the launch of insert able cardiac monitor, Jot Dx in U.S. The device helps clinicians to view abnormal heart rhythm and allows for remote detection and improved diagnosis accuracy of cardiac arrhythmia. This will help the company to further acquire the market in coming years
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.