Mercado de combinação de medicamentos e dispositivos da América do Norte espera-se que cresça com o CAGR de 10,9% no período de previsão de 2021 a 2028. Os anos considerados para estudo são os mencionados abaixo.
Acesse o relatório completo @ https://www.databridgemarketresearch.com/reports/north-america-drug-device-combination-market
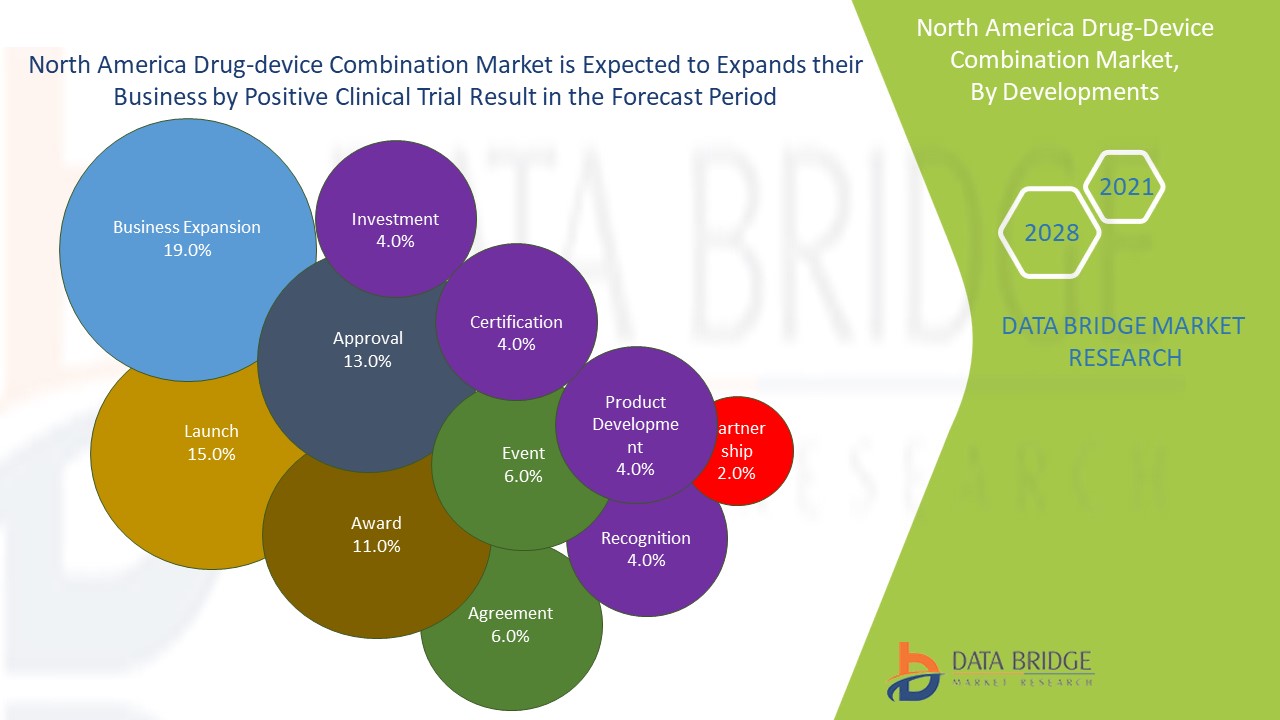
O mercado de combinação de dispositivos de drogas da América do Norte é um mercado altamente consolidado, que inclui um número específico de participantes-chave. O mercado tem testemunhado maiores desenvolvimentos estratégicos devido ao cenário de mercado favorável.
Os principais players que negociam no mercado de combinação de dispositivos de drogas da América do Norte estão introduzindo uma forte gama de fornecedores de produtos, juntamente com o lançamento de novos produtos e adotando iniciativas estratégicas como aquisição, acordo, expansão de negócios, premiação e reconhecimento no mercado. Isso ajudou as empresas a maximizar as vendas com um portfólio aprimorado de produtos.
Por exemplo:
- Em setembro de 2018, a Amgen Inc. recebeu aprovação de comercialização do Ministério Japonês da Saúde, Trabalho e Bem-Estar para Blincyto (blinatumomab). O uso de primeira linha de Blincyto (blinatumomab) é tratar leucemia linfoblástica aguda de células B recidivante ou refratária. Esta aprovação acelerou a posição da empresa no mercado japonês.
A Janssen Pharmaceuticals, Inc, (uma subsidiária da Johnson & Johnson Services, Inc.), é o player dominante no mercado de combinação de medicamentos e dispositivos na América do Norte. Os outros principais players existentes no mercado são Mediprint, OcuMedic, Medtronic, GlaxoSmithKline Plc, BD, Janssen Pharmaceuticals, Inc. (uma subsidiária da Johnson & Johnson Services, Inc.), Bayer AG, Micron Biomedical, Inc., Otsuka America Pharmaceutical , Inc., VAXXAS, Propeller Health, Raphas Co., Ltd., EOFLOW CO., LTD., Insulet Corporation, TheraJect, Zimmer Biomet, Amgen Inc., Eitan Medical entre outros.
Amgen Inc. fundada no ano de 1980, com sede em Thousand Oaks, Califórnia, Estados Unidos. A Empresa está altamente focada na redução do custo dos cuidados de saúde e no aumento da sua presença no mercado. Além disso, a empresa está se concentrando em pesquisa e desenvolvimento de novas terapêuticas humanas para o tratamento de doenças graves. A empresa opera seus negócios por meio de um segmento de negócios que inclui terapêutica humana, que é o segmento focado no mercado. A empresa possui diversas categorias de produtos que são Aimovig, Aranesp, AVSOLA, BLINCYTO, Corlanor, Repatha, Sensipar, Prolia e Neulasta entre outras em que Repatha e Neulasta são as categorias focadas no mercado.
Por exemplo,
- Em janeiro de 2020, a Amgen Inc. anunciou a colaboração estratégica bem-sucedida com a BeiGene. Prevê-se que esta colaboração melhore a presença da empresa no mercado oncológico da China nos próximos anos.
A empresa está presente na América do Norte e no resto do mundo. A empresa também possui várias subsidiárias, incluindo Alantos Pharmaceuticals Holdings (Delaware), Amgen Canada Inc. (Ontário), Amgen Fremont Inc. Amgen Ilac Ticaret Limited Sirketi (Turquia) e Amgen SAS (França), entre outros.
Janssen Pharmaceuticals, Inc, (uma subsidiária da Johnson & Johnson Services, Inc.)
(uma subsidiária da Johnson & Johnson Services, Inc.), fundada em 1953, com sede na Bélgica. A empresa está focada em diversas áreas da medicina, como cardiovascular e metabolismo, imunologia, doenças infecciosas e vacinas, neurociência, oncologia e hipertensão pulmonar. A empresa possui várias categorias de produtos, incluindo SmartJect, TREMFYA e SmartJect, TREMFYA são a categoria focada no mercado.
Por exemplo,
- Em outubro de 2009, Simponi (Golimumab) recebeu aprovação europeia como anti-TNF subcutâneo uma vez por mês para tratamento de artrite reumatóide, artrite psoriática e espondilite anquilosante com o novo autoinjetor Smartject. Isto fortaleceu a presença da empresa na Europa e impulsionou o crescimento do mercado.
A empresa tem ampla presença nas Américas, Europa, Oriente Médio e África, Ásia-Pacífico. A empresa também possui várias subsidiárias, incluindo Janssen-Cilag A/S (Dinamarca), Janssen Pharmaceutica (Pty.) Ltd. (África do Sul), Johnson & Johnson del Perú SA (Peru), Janssen-Cilag Farmacêutica Ltda. (Brasil) e Janssen Pharmaceuticals, Inc. (EUA), entre outros.
GlaxoSmithKline Plc.
GlaxoSmithKline Plc fundada no ano de 1999, com sede na Grande Londres, Reino Unido. O foco principal da empresa é uma empresa de saúde, que se dedica à pesquisa, desenvolvimento e fabricação de medicamentos farmacêuticos, vacinas e produtos de saúde ao consumidor. A empresa opera seus negócios por meio de vários segmentos de negócios, incluindo produtos farmacêuticos, vacinas e cuidados de saúde ao consumidor, nos quais os produtos farmacêuticos são o segmento focado no mercado. A empresa possui diversas categorias de produtos que são Medicamentos e Vacinas Prescritas, Produtos de Saúde de Consumo e ViiV Healthcare, nas quais Medicamentos e Vacinas Prescritas são a categoria focada no mercado.
Por exemplo,
- Em outubro de 2020, a Glaxosmithkline Plc anunciou que a Agência Europeia de Medicamentos (EMA) aceitou submissões regulatórias buscando aprovação para o uso do Nucala biológico anti-IL5 (mepolizumabe) em três condições adicionais: síndrome hipereosinofílica (HES), rinossinusite crônica com pólipos nasais ( RSCcPN) e granulomatose eosinofílica com poliangeíte (EGPA). Isso aumentou a receita da empresa, atraindo assim mais clientes para sua empresa.
A empresa está presente na América do Norte, Europa, Ásia-Pacífico, América do Sul, Oriente Médio e África. A empresa também possui várias subsidiárias, incluindo GlaxoSmithKline Pharmaceuticals Ltd. (Índia), GlaxoSmithKline Paquistão (Paquistão), Tesaro (EUA), GlaxoSmithKline LLC (Canadá) e Alacer Corp.