A carga de doenças infecciosas está a aumentar rapidamente em toda a Europa. Vários tipos de microrganismos patogénicos estão associados a doenças infeciosas, como vírus, fungos, bactérias e parasitas. À medida que as doenças infeciosas se tornam mais frequentes e complexas, a necessidade de ferramentas de diagnóstico mais eficientes, rápidas e abrangentes torna-se crítica. O diagnóstico multiplex sindrómico oferece uma solução ao permitir a deteção simultânea de vários agentes patogénicos num único teste, uma capacidade que se está a tornar cada vez mais essencial face a doenças infeciosas novas e reemergentes. Aumento de casos de doenças infeciosas, o que está a aumentar a taxa de diagnóstico multiplex sindrómico, devido ao qual a crescente prevalência de doenças infeciosas está a impulsionar o crescimento do mercado.
Aceda ao relatório completo em https://www.databridgemarketresearch.com/reports/europe-syndromic-multiplex-diagnostic-market
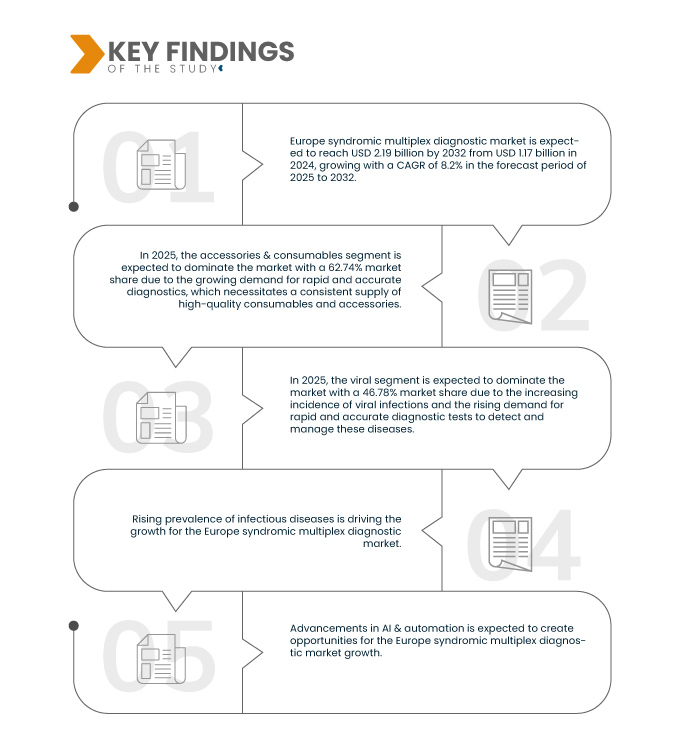
A Data Bridge Market Research analisa que o mercado europeu de diagnóstico multiplex sindrómico deverá atingir os 2,19 mil milhões de dólares até 2032, face aos 1,17 mil milhões de dólares em 2024, crescendo a um CAGR de 8,2% no período previsto de 2025 a 2032.
Principais conclusões do estudo
Adoção crescente de técnicas de diagnóstico molecular
O diagnóstico molecular é um campo em rápida evolução e transformação que está a revolucionar a deteção, o tratamento e a investigação de doenças. Permite a identificação precisa de doenças infeciosas, geralmente em fase inicial. No diagnóstico molecular , ensaios de painel multiplex PCR (Reação em Cadeia da Polimerase) são usados para diagnosticar doenças infecciosas detectando simultaneamente material genético de vários patógenos. Estes ensaios podem identificar diferentes tipos de microrganismos, incluindo fungos, parasitas, bactérias e vírus, a vários níveis taxonómicos.
Âmbito do Relatório e Segmentação de Mercado
Métrica de Reporte
|
Detalhes
|
Período de previsão
|
2025 a 2032
|
Ano base
|
2024
|
Ano Histórico
|
2023 (Personalizável 2013-2017)
|
Unidades quantitativas
|
Receita em biliões de dólares americanos
|
Segmentos abrangidos
|
Tipo de produto (acessórios e consumíveis, instrumentos e software e serviços), tipo de infecção (viral, bacteriana, fúngica, parasitária e outras), doença (infecções respiratórias, gastroenterite, infecções sexualmente transmissíveis , meningite, infecções infantis, imunossupressão, febre tropical, hepatite, infecções oculares e outras doenças), utilizador final (hospitais, laboratórios clínicos, consultórios médicos, empresas farmacêuticas e de biotecnologia, institutos de investigação e outros), canal de distribuição (licitação directa, vendas no retalho e outros)
|
Países abrangidos
|
Alemanha, Reino Unido, França, Itália, Espanha, Rússia, Países Baixos, Suíça, Turquia, Bélgica, Suécia, Polónia, Dinamarca, Noruega, Finlândia e resto da Europa
|
Participantes do mercado abrangidos
|
F. Hoffmann-La Roche Ltd (Suíça), DiaSorin SpA (Itália), BIOMÉRIEUX (França), Thermo Fisher Scientific Inc. (EUA), QIAGEN (Holanda), BD Veritor (BD) (EUA), Bio-Rad Laboratories, Inc. (EUA), Danaher Corporation (EUA), Seegene Inc. (Coreia do Sul), Hologic, Inc. (EUA), Bosch Healthcare Solutions GmbH (Alemanha), Abbott (EUA), QuidelOrtho Corporation (EUA), Biocartis (Bélgica) e PathoFinder (Holanda), entre outros
|
Pontos de dados abordados no relatório
|
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços e estrutura regulamentar.
|
Análise de Segmentos
O mercado europeu de diagnóstico multiplex sindrómico está categorizado em cinco segmentos notáveis com base no tipo de produto, tipo de infeção, doença, utilizador final e canal de distribuição.
- Com base no tipo de produto, o mercado europeu de diagnóstico multiplex sindrómico está segmentado em acessórios e consumíveis, instrumentos e software e serviços
Em 2025, prevê-se que o segmento de acessórios e consumíveis domine o mercado europeu de diagnóstico multiplex sindrómico
Em 2025, prevê-se que o segmento dos acessórios e consumíveis domine o mercado com uma quota de mercado de 62,74% devido à crescente procura de diagnósticos rápidos e precisos, o que exige um fornecimento consistente de consumíveis e acessórios de alta qualidade.
- Com base no tipo de infeção, o mercado europeu de diagnóstico multiplex sindrómico está segmentado em vírus, bactérias, fungos, parasitas e outros
Em 2025, prevê-se que o segmento viral domine o mercado europeu de diagnóstico multiplex sindrómico
Em 2025, prevê-se que o segmento viral domine o mercado com uma quota de mercado de 46,78% devido à crescente incidência de infeções virais e à crescente procura de testes de diagnóstico rápidos e precisos para detetar e controlar estas doenças.
- Com base na doença, o mercado europeu de diagnóstico multiplex sindrómico está segmentado em infeções respiratórias, gastroenterite, infeções sexualmente transmissíveis, meningite, infeções infantis, imunossupressão, febre tropical, hepatite, infeções oculares e outras doenças. Em 2025, prevê-se que o segmento das infeções respiratórias domine o mercado com uma quota de mercado de 33,94%
- Com base no utilizador final, o mercado europeu de diagnóstico multiplex sindrómico está segmentado em hospitais, laboratórios clínicos, consultórios médicos, empresas farmacêuticas e de biotecnologia, institutos de investigação e outros. Em 2025, prevê-se que o segmento hospitalar domine o mercado com uma quota de mercado de 45,05%
- Com base no canal de distribuição, o mercado europeu de diagnóstico multiplex sindrómico está segmentado em licitação direta, vendas a retalho e outros. Em 2025, prevê-se que o segmento de licitação direta domine o mercado com uma quota de mercado de 70,37%
Principais jogadores
A Data Bridge Market Research analisa a F. Hoffmann-La Roche Ltd. (Suíça), a DiaSorin SpA (Itália), a BIOMÉRIEUX (França), a Thermo Fisher Scientific Inc. (EUA) e a QIAGEN (Holanda) como as principais empresas do mercado de diagnóstico multiplex sindrómico na Europa.
Desenvolvimentos de mercado
- Em novembro de 2024, a Roche assinou um acordo para adquirir a Poseida Therapeutics por aproximadamente mil milhões de dólares. Esta aquisição aumenta as capacidades da Roche em terapias celulares alogénicas, particularmente para oncologia, imunologia e neurologia. O objetivo é acelerar o desenvolvimento de terapias CAR-T prontas a usar, beneficiando os doentes ao aumentar o acesso ao tratamento e melhorar os resultados clínicos. A transação deverá estar concluída no primeiro trimestre de 2025
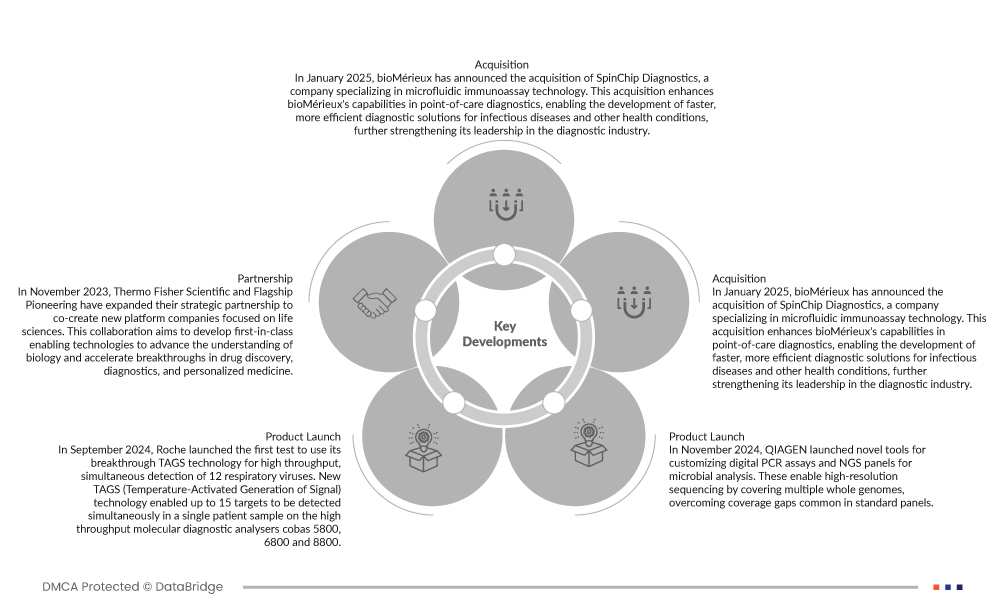
- Em setembro de 2024, a Roche lançou o primeiro teste a utilizar a sua inovadora tecnologia TAGS para a deteção simultânea de alto rendimento de 12 vírus respiratórios. A nova tecnologia TAGS (geração de sinal ativada por temperatura) permitiu a deteção simultânea de até 15 alvos numa única amostra de paciente nos analisadores de diagnóstico molecular de alto rendimento cobas 5800, 6800 e 8800
- Em maio de 2023, a DiaSorin SpA reforça a sua parceria com a MeMed ao assinar um acordo de distribuição para o teste MeMed BV em Itália. Este teste, integrado na IA, diferencia as infeções bacterianas das virais, auxiliando nas decisões sobre os antibióticos. Validado globalmente, melhora a gestão do doente e apoia os esforços para combater a resistência antimicrobiana
- Em janeiro de 2025, a bioMérieux anunciou a aquisição da SpinChip Diagnostics, uma empresa especializada em tecnologia de imunoensaio microfluídico. Esta aquisição melhora as capacidades da bioMérieux em diagnósticos no local de atendimento, permitindo o desenvolvimento de soluções de diagnóstico mais rápidas e eficientes para doenças infeciosas e outras condições de saúde, reforçando ainda mais a sua liderança no setor do diagnóstico.
- Em fevereiro de 2025, a Thermo Fisher Scientific anunciou a aquisição do negócio de purificação e filtragem da Solventum. A mudança expande as capacidades da Thermo Fisher no fornecimento de soluções de filtragem avançadas e de alta qualidade para aplicações críticas. Esta aquisição estratégica fortalece o portefólio da Thermo Fisher, melhorando as suas ofertas para apoiar clientes de investigação científica, saúde e indústria em todo o mundo.
Análise Regional
Os países abrangidos pelo mercado são a Alemanha, Reino Unido, França, Itália, Espanha, Rússia, Países Baixos, Suíça, Turquia, Bélgica, Suécia, Polónia, Dinamarca, Noruega, Finlândia e resto da Europa.
De acordo com a análise de pesquisa de mercado da Data Bridge:
Espera-se que a Alemanha domine e seja o país com o crescimento mais rápido no mercado europeu de diagnóstico multiplex sindrómico
A Alemanha deverá liderar e crescer rapidamente no mercado devido à crescente procura de resultados de diagnóstico rápidos e precisos e às iniciativas estratégicas tomadas pelos participantes do mercado.
Para obter informações mais detalhadas sobre o mercado, clique aqui – https://www.databridgemarketresearch.com/reports/europe-syndromic-multiplex-diagnostic-market